Abstract
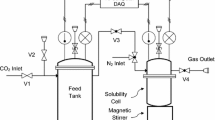
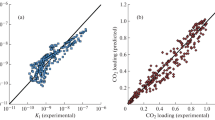
The modified Kent-Eisenberg model was used to predict the solubility of carbon dioxide in aqueous 2-amino-2-methyl-1,3-propanediol (AMPD) solutions over a wide range of solvent concentration (10-30 mass %), temperature (30-60 °C), and partial pressure of carbon dioxide (5-1,100 kPa). For more accurate prediction, a new set of experimental data of this system was also presented and used in model calculation. The predicted results by the modified Kent-Eisenberg model were found to be in good agreement with the experimental data. The equilibrium constant, K1, which represented the deprotonation reaction of AMPD, was expressed as a function of not only temperature but also loading capacity and amine concentration.
Similar content being viewed by others
References
Austgen, D. M., Rochelle, G. T. and Chen, C. C., “Model of Vapor-Liquid Equilibria for Aqueous Acid Gas-Alkanolamine Systems. 2. Representation of H2S and CO2 Solubility in Aqueous MDEA and CO2 Solubility in Aqueous Mixtures of MDEA with MEA or DEA,”Ind. Eng. Chem. Res.,30, 543 (1991).
Baek, J.-I. and Yoon, J.-H., “Solubility of Carbon Dioxide in Aqueous Solutions of 2-Amino-2-methyl-1,3-propanediol,”J. Chem. Eng. Data,43, 635 (1998).
Chakma, A. and Meisen, A., “Solubility of CO2 in Aqueous Methyldiethanolamine and N,N,-Bis-(hydroxyethyl)piperazine Solutions,”Ind. Eng. Chem. Res.,26, 2461 (1987).
Deshmukh, R. D. and Mather, A. E., “A Mathematical Model for Equilibrium Solubility of Hydrogen Sulfide and Carbon Dioxide in Aqueous Alkanolamine Solutions,”Chem. Eng. Sci.,36, 355 (1981).
Hu, W. and Chakma, A., “Modelling of Equilibrium Solubility of CO2 and H2S in Aqueous Amino Methyl Propanol (AMP) Solutions,”Chem. Eng. Commun.,94, 53 (1990).
Isaacs, E. E., Otto, F. D. and Mather, A. E., “Solubility of Mixtures of H2S and CO2 in a Monoethanolamine Solutions at Low Partial Pressures,”J. Chem. Eng. Data,25, 118 (1980).
Jou, F.-Y., Mather, A. E. and Otto, F. D., “Solubility of H2S and CO2 in Aqueous Methyldiethanolamine Solutions,”Ind. Eng. Chem. Process Des. Dev.,21, 539 (1982).
Jou, F.-Y., Mather, A. E. and Otto, F. D., “The Solubility of CO2 in a 30 Mass Percent Monoethanolamine Solution,”Can. J. Chem. Eng.,73, 140 (1995).
Jou, F.-Y., Otto, F. D. and Mather, A. E., “Vapor-Liquid Equilibrium of Carbon Dioxide in Aqueous Mixtures of Monoethanolamine and Methyldiethanolamine,”Ind. Eng. Chem. Res.,33, 2002 (1994).
Kent, R. L. and Eisenberg, B., “Better Data for Amine Treating,”Hydrocarbon Process,55, 87 (1976).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baek, JI., Yoon, JH. & Eum, HM. Prediction of equilibrium solubility of Carbon Dioxide in aqueous 2-amino-2-methyl-1,3-propanediol Solutions. Korean J. Chem. Eng. 17, 484–487 (2000). https://doi.org/10.1007/BF02706866
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02706866