Abstract
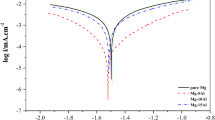
A series of electrochemical experiments on Al alloys were undertaken to determine their optimum protection potentials in seawater. With 1050 and 5456 alloys, passive films form during anodic polarization but are destroyed by the Cl in seawater, only to regrow as a result of the self-healing capacity of aluminum. The current density of 5456 Al alloy proved to be lower than that of 1050 as a whole. Any shift to more anodic or cathodic conditions in the potential range of-1.5∼-0.68 V resulted in a sudden increase in current density. Current densities in the high-strength 7075 Al alloy showed the greatest values. In contrast, the current densities of 5456 alloy, known to have excellent corrosion resistance in seawater, were the lowest in the range of -0.70∼-1.3 V, and we concluded that this potential range offered optimal protection.
Similar content being viewed by others
References
Cho, J. H.,Korea research institute of medium and small shipbuilding,10, 6 (2004).
Inoue, T. and Oki, T., “Characterization of passivation film on surface of aluminum alloy by heat-treatment,”Journal of Japan Institute of Heat Treatment,34(2), 110 (1994).
Jang, S. K., Lee, D. C., Kim, S. J., Jeon, J. I. and Kim, S. H., “Investigation of macrostructures and properties for friction stir welded 1050 aluminum alloy sheet,”The proceeding of Korean Society of Marine Engineers, 139 (2004).
Jeon, D. H.,Control of corrosion and protection, 316, Il-Joong publishing Co. (1985).
Kanematsu, H., Okido, M. and Oki, T., “Electrochemical study on stress corrosion cracking of Al-Zn-Mg alloy,”Journal of Japan Institute of Light Metals,36(3), 125 (1986).
Kanematsu, H., Okido, M. and Oki, T., “The effects of heat treatments on the SCC susceptibility of Al-Zn-Mg alloy,”Journal of Japan Institute of Light Metals,36(5), 255 (1986).
Kang, B. Y. and Cho, J. H., “Consideration for structure and fabrication procedure of Al boat,”The Korean Journal of Welding Society,22(3), 39 (2004).
Kim, S. J. and Moon, K. M., “Hydrogen embrittlement properties of heat affected zone of high strength steel in shielded metal arc welding,”Metals and Materials International,8(4), 395 (2002).
Kim, S. J. and Moon, K. M., “The relationship between corrosion protection and hydrogen embrittlement properties of HAZ in flux cored arc welding,”Metals and Materials International,8(4), 387 (2002).
Kim, S. J., Okido, M. and Moon, K. M., “An electrochemical study of cathodic protection of steel used for marine structures,”Korean J. Chem. Eng.,20, 560 (2003).
Kim, S. J., Okido, M. and Moon, K. M., “The electrochemical study on mechanical and hydrogen embrittlement properties of HAZ part as a function of post-weld heat treatment in SMAW,”Surface and coatings Technology,169–170, 163 (2003).
Pourbaix, M., “Atlas of electrochemical equilibria,”NACE, 168 (1974).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SJ., Ko, JY. Electrochemical properties of Al and Al alloys relevant to corrosion protection in seawater environments. Korean J. Chem. Eng. 23, 847–853 (2006). https://doi.org/10.1007/BF02705939
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705939