Abstract
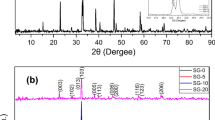
Because of the presence of water vapor, YBa2Cu3O7-x degrades easily in the atmosphere and thus very difficult to put it to practical use. When Cu is partially substituted with Ag, however, there is not much a decrease in the critical temperature (Tc) : Tc is 89 K compared with 91 K without substitution. Moreover, partial substitution of Ag increases density, hardness, and superconducting particle size and above all improves considerably its stability in water. In this study YBa2Cu3O7-x and YBa2Cu3-yAgyO7-x as a result of a partial substitution of Ag for Cu were synthesized by pyrophoric method. We investigated their stabilities in water by XRD, SEM, and EPMA after immersing samples in distilled water for 3 hours. We can see that YBa2Cu2.94Ag0.06O7-x was the largest particle size and anticorrosion behavior.
Similar content being viewed by others
References
Barns, R. L. and Laudise, R. A., “Stability of Superconducting YBa2- Cu3O7 in the Presence of Water”,Appl. Phys. Lett.,51, 1373 (1987).
Bansal, N. P. and Sandkuhl, A. L., “Chemical Durability of High-temperature Superconductor YBa2Cu3O7-x in Aqueous Environments”,Appl. Phys. Lett.,52(4), 323(1988).
Bhattacharya, D., Pathak, L. C., Mishra, S. K., Sen, D. and Chopra, K. L., “Pyrophoric Synthesis Technique for Multicomponent High-temperature Superconductors”,Appl. Phys. Lett.,57(20), 2145 (1990).
Chu, C. W., Hor, P. H., Meng, R. L., Gao, L., Huang, Z. J. and Wang, Y. Q., “Evidence for Superconductivity above 40K in the La-Ba-Cu-O Compound System”,Phys. Rev. Lett,58(4), 405(1987).
Dwir, D., Affronte, M. and Pavuna, D., “Evidence for Enhancement of Critical Current by Intergrain Ag in YBaCuO-Ag Ceramics”,Appl. Phys. Lett.,55(4), 399(1989).
Eibschütz, M., Lines, M. E., Reiff, W. M., van Dover, B., Waszczak, J. V., Zahurak, S. and Felder, R. J., “Direct Observation of Gold Valance Chance as a Function of Oxygen Stoichiometry in YBa2- Cu3-yAuyO7-δ”,Appl. Phys. Lett.,62(15), 1827(1993).
Kim, Y. S., Park, J. S. and Shin, H. S., “Preparation and Properties of YBa2Cu3O7-x High-Tc Superconductors by Pyrophoric Synthetic Method”,Korea Chonbuk Nation Univ.(Research of Eng.),24, 149(1993).
Kumar, D., Sharon, M., Pinto, R., Apte, P. R., Pai, S. P., Purandare, S. C., Gupta, L. C. and Vijayaraghavan, R., “Large Critical Currents and Improved Epitaxy of Laser Ablated Ag-doped YBa2Cu3- O7-δ Thin Films”,Appl. Phys. Lett.,62, 3522(1993).
Muromachi, E. T., Uchida, Y. and Kato, K., “Superconductivity of YBa2Cu3-xMxOy (M = Co, Fe, Ni, Zn”,Jpn. J. Appl. Phys.,26(12), L2087(1987).
Nakada, I., Sato, S., Oda, Y. and Kohara, T., “Two Crystal Phases in the Superconducting Ba-Y-Cu-O System and Their Reactivity to Water”,J. Appl. Phys.,26, L697 (1987).
Nobuhito, I., Funihiko, S., Hisao, I. and Gin-ya, A., “Critical Current Characteristics of YBa2Cu3O7-x-Ag Composite”,Jpn. J. Appl. Phys. 28(4), L580(1989).
Noguchi, S., Inoue, J., Okuda, K., Maeno, Y. and Fujita, T., “Magnetic Susceptibility of Fe-Doped YBa2Cu3O7-δ”,Jpn. J. Appl. Phys.,27(3), L390(1988).
Park, J. H., Kim, B. C. and Song, J. T., “A Study on the Microstructure and Properties of Y-Ba-Cu-O/Ag Composite High T, Superconductor Prepared by Sinter-forging Process”,Kor. J. of Mat. Res.,4(1), 37 (1994).
Sengupta, S., Shi, D., Wang, Z., Biondo, A. C., Balachandran, U. and Goretta, K. C., “Effect of Y2BaCuO5 Precipitates”,Physica C.,199, 43 (1992).
Siegrist, T., Schneemeyer, L. F., Waszczak, J.V., Singh, N. P., Opila, R. L., Batlogg, B., Rupp, L. W. and Murphy, D. W., “Aluminum Substitution in Ba2YCu3O7”,Phys. Rev.,B36(16), 8365 (1987).
Weast, R. C.: CRC Handbook of Chemistry and Physics, 63rd ed. (CRC, Boca Raton, FL, 1982–1983).
Yan, M. F., Barns, R. L., O’Bryan, H. M., Jr., Gallagher, P. K., Sherwood, R. C. and Jin, S., “Water Interaction with the Superconducting YBa2Cu3O7 Phase”,Appl. Phys. Lett.,51, 532 (1987).
Yang, Y.M., Out, P., Jhao, B.R., Jhao, Y.Y., Li, L., Ran, Q. Z. and Jin, R. Y., “Characterization of YBa2Cu3O7-x Bulk Samples Prepared by Citrate Synthesis and Solid-state Reaction”,J. Appl. Phys.,66(1), 312 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, YS., Park, JS., Chung, BW. et al. Corrosion behavior of high-temperature superconductor YBa2Cu3-yAgyO7-x in the presence of water. Korean J. Chem. Eng. 12, 563–566 (1995). https://doi.org/10.1007/BF02705860
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705860