Abstract
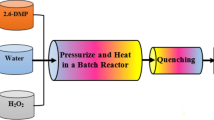
2,4-Dichlorophenol (2,4-DCP), as a halogenated model pollutant, was decomposed by using supercritical water oxidation (SCWO) in a batch reactor made of Hastelloy C-276. SCWO experiments for 2,4-DCP decomposition were performed in the range of 380–420 °C, 230–280 bar and 0.074-0.221 mol/L H2O2. The effect of oxidant concentration on decomposition rate and efficiency was significant near the critical temperature of 380 °C. However, the role of the oxidant concentration in the SCWO process decreased with an increase in temperature; also, excess oxidant played a key role in quite significantly decreasing the activation energy of 2,4-DCP oxidation. Variation of the reaction rate by the change of pressure was negligible even at a near critical temperature. The kinetic rate for the decomposition of 2,4-DCP in the SCWO process was well described by a simple first-order kinetic and global reaction rate model. From the SCWO experiments, the various intermediates identified with a GC/MS implied that the first reaction pathway for 2,4-DCP decomposition led to dimers such as dichlorophenoxyphenols, and the second led to single-ring and ring-opening products.
Similar content being viewed by others
References
Armenante, P. M., Kafkewitz, D., Lewandowski, G A. and Jou, C. J., “Anaerobic-Aerobic Treatment of Halogenated Phenolic Compounds,”Wat. Res.,30, 681 (1999).
Cocero, M. J., Alonso, E. and Sanz, M. T., “Supercritical Water Oxidation Process under Energetically Self-sufficient Operation,”J. Supercrit. Fluids,24, 37 (2002).
Connoly, J. F., “Solubility of Hydrocarbons in Water Near the Critical Temperature,”Chem. Eng. J.,13, 11 (1996).
Gopalan, S. and Savage, P. E. A., “Reaction Network Model for Phenol Oxidation in Supercritical Water,”AIChE J.,41, 1864 (1995).
Christopher, J., Martino and Savage, P. E., “Total Organic Carbon Disappearance Kinetics for the Supercritical Water Oxidation of Mono-substituted Phenols,”Environ. Sci. Technol.,33, 1911 (1999).
Japas, M. L. and Franck, E. U., “High Pressure Phase Equilibria and PVT-data of the Water-oxygen System Including Water-air to 673 K and 25 MPa,”Phys. Chem.,89, 1268 (1985).
Jianli, Y. and Savage, P. E., “Kinetics of Catalytic Supercritical Water Oxidation of Phenol over TiO2,”Environ. Sci. Technol.,34, 3191 (2000).
Juan, R. P., Lopez, J., Nebot, E. and Martinez, E., “Elimination of Cutting Oil Wastes by Promoted Hydrothermal Oxidation,”J. Hazard. Mater.,88, 95 (2001).
Konys, J., Fodi, S., Hausselt, J., Schmidt, H. and Casal, V., “Corrosion of High-temperature Alloys in Chloride-containing Supercritical Water Oxidation Systems,”Corrosion,55, 45 (1999).
Kritzer, P., Boukis, N. and Dinjus, E., “An Assessment of Supercritical Water Oxidation (SCWO); Existing Problems, Possible Solution and New Reactor Concepts,”Corrosion,54, 824 (1998).
Lee, G., Nunoura, T., Matsumura, Y. and Yamamoto, K., “Comparison of the Effects of the Addition of NaOH on the Decomposition of 2-Chlorophenol and Phenol in Supercritical Water and under Super-critical Water Oxidation Conditions,”J. Supercrit. Fluids,24, 239 (2002).
Lee, H. C., In, J. H., Hwang, K. Y. and Lee, C. H., “Decomposition of Ethylenediaminetetraacetic Acid (EDTA) by Supercritical Water Oxidation,” Ind.Eng, Chem. Res.,43, 3223 (2004).
Lee, H. C., Kim, J. H., In, J. H. and Lee, C. H., “NaFeEDTA Decomposition and Hematite Nanoparticle Formation in Supercritical Water Oxidation,”Ind. Eng, Chem. Res.,17, 6615 (2005).
Lin, K. S., Wang, H. P. and Yang, Y.W., “Supercritical Water Oxidation of 2-Chlorophenol Effected by Li+ and CuO/Zeolites,”Chemosphere,39, 1385 (1999).
Martino, C. J. and Savage, P. E., “Total Organic Carbon Disappearance Kinetics for the Supercritical Water Oxidation of Monosubstituted Phenols,”Environ. Sci. Techno.,33, 1911 (1999).
Matsumura, Y., Nunoura, T., Urase, T. and Yamamoto, K., “Supercritical Water Oxidation of High Concentrations of Phenol,”J. Hazard. Mater.,73, 245 (2000).
Mitton, D. B., Yoon, J. H., Cline, J. A., Kim, H. S., Eliaz, N. and Latanision, R. M., “Corrosion Behavior of Nickel-Based Alloys in Supercritical Water Oxidation Systems,”Ind. Eng. Chem. Res.,39, 4689 (2000).
Mitton, D. B., Yoon, J. H. and Latanision, R. M., “An Overview of Corrosion Phenomena in SCWO Systems for Hazardous Waste Destruction,”Zairyo-to-Kankyo,49, 130 (2000).
Modell, M.,Standard Hand-book for Hazardous Wastes Treatment and Disposal, H.M. Freeman, ED. (1986).
Motonobu, G., Daisuke, S., Akio, K. and Tsutomu H., “Kinetic Analysis for Ammonia Decomposition in Supercritical Water Oxidation of Sewage Sludge,”Ind. Eng. Chem. Res.,38, 4500 (1999).
Motonobu, G., Takatsugu, N., Akio, K. and Tsutomu, H., “Kinetic Analysis for Destruction of Municipal Sewage Sludge and Alcohol Distillery Wastewater by Supercritical Water Oxidation,”Ind. Eng. Chem. Res.,38, 1863 (1999).
Ormad, M. P., Ovelleiro, J. L. and Kiwi, J., “Photocatalytic Degradation of Concentrated Solutions of2,4-Dichlorophenol using Low Energy Light: Identification of Intermediates,”Appl. Catal. B: Environ.,32, 157 (2001).
Peter, K. and Eckhard, D., “An Assessment of Supercritical Water Oxidation (SCWO): Existing Problems, Possible Solutions and New Reactor Concepts,”Chem. Eng. J.,83, 207 (2001).
Portela, J. R., Nebot, E. and Ossa, E. M., “Kinetic Comparison Between Subcritical and Supercritical Water Oxidation of Phenol,”Chem. Eng. J.,81, 287 (2001).
Quan, X., Shi, H., Wang, J. and Qian, Y., “Biodegradation of 2,4-Di-chlorophenol in Sequencing Batch Reactors Augmented with Immobilized Mixed Culture,”Chemosphere,50, 1069 (2003).
Ruokang, L., Phillip, E. S. and David, S., “2-Chlorophenol Oxidation in Supercritical Water: Global Kinetics and Reaction Products,”AIChE J.,39, 178 (1993).
Sako, T., Sugeta, T., Otake, K., Sato, M., Tsugumi, M., Hiaki, T. and Hongo, M., “Decomposition of Dioxin in Fly Ash with Supercritical Water Oxidation,”J. Chem. Eng. Jpn.,30, 744 (1997).
Savage, P. E., Yu, J., Stylski, N. and Brock, E. E., “Kinetics and Mechanism of Methane Oxidation in Supercritical Water,”J. Supercrit. Fluids,12, 141 (1998).
Takehiro, M., Motonobu, G., Akio, K. and Tsutomu, H., “Supercritical Water Oxidation of a Model Municipal Solid Waste,”Ind. Eng. Chem. Res.,39, 2807 (2000).
Tang, W. Z. and Huang, C. P., “Photocatalyzed Oxidation Pathways of 2,4-Dichlorophenol by CdS in Basic and Acidic Aqueous Solutions,”Wat. Res.,29, 745 (1995).
Uematsu, M. and Franf, E. U., “Static Dielectric Constant of Water and Stream,”J. Phys. Chem.,9, 1291 (1980).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, HC., In, JH., Kim, JH. et al. Kinetic analysis for decomposition of 2,4-dichlorophenol by supercritical water oxidation. Korean J. Chem. Eng. 22, 882–888 (2005). https://doi.org/10.1007/BF02705669
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705669