Abstract
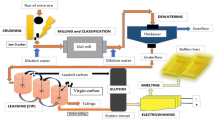
Chemical decontamination is considered to be an effective method for reduction of radiation level by dissolution of radioactive corrosion products and metal oxidizing films existing in the primary system of a nuclear power plant. In this study, the process efficiencies of two chemical decontamination processes (Methods 1 and 2) having different reduction steps were investigated through the operation of a semi-pilot scale decontamination equipment as a continuous work. The reduction step for Method 1 employed an adsorbent with an oxygen source, while a reductant (oxalic acid) was used in the reduction step for Method 2. The dissolution and removal efficiencies of metal species and organic compounds in Method 2 were higher than those in Method 1, implying that oxalic acid in the reduction step increased the process efficiency, their complexes of metal species easily being removed in the decomposition/cleanup step. It was shown that the process employing chemical reduction showed higher dissolution and removal efficiencies rather than the process by the physical adsorption on the adsorbent surface through decontamination processes with different reduction step.
Similar content being viewed by others
References
Ayres, J. A.,Decontamination of Nuclear Reactors and Equipment, Ronald Press, New York (1970).
Choi, W., Kim, S., Cho, S., Yoo, H. I. and Kim, M. H., “Photocatalytic Reactivity and Diffusing OH Radicals in the Reaction Medium Containing TiO2 Particles,”Korean J. Chem. Eng.,18, 898 (2001).
Dastgheib, S. A. and Rockstraw, D. A., “Pecan Shell Activated Carbon: Synthesis, Characterization, and Application for the Removal of Copper from Aqueous Solution,”Carbon,39, 1849 (2001).
Faur-Brasquet, C., Kadiruvelu, K. and Le Chloirec, P., “Removal of Metal Ions from Aqueous Solution by Adsorption onto Activated Carbon Cloths: Adsorption Competition with Organic Matter,”Carbon,40, 2387 (1996).
Frim, J. A., Rathman, J. F. and Weavers, L. K., “Sonochemical Destruction of Free and Metal Binding Ethylenediaminetetraacetic Acid,”Water Research,37, 3155 (2003).
Gregg, S. J. and Sing, K. S. W.,Adsorption, Surface Area and Porosity, Academic Press, London (1982).
Juang, R. S. and Wang, Y.C., “Use of Complexing Agents for Effective Ion-Exchange Separation of Co(II)/Ni(II) from Aqueous Solution,”Water Research,37, 845 (2003).
Kahlili, N. R., Campbell, M., Sandi, G. and Golas, J., “Production of Micro- and Mesoporous Activated Carbon from Paper Mill Sludge I. Effect of Zinc Chloride Activation,”Carbon,38, 1905 (2000).
Kim, H. J., Moon, H. and Park, H. C., “Multicomponent Adsorption Equilibrium of Phenols on Activated Carbon-Application of Ideal Adsorbed Solution (IAS) Theory,”Korean J. Chem. Eng.,2, 181 (1985).
Kim, K., Lee, H. J., Choi, M., Kang, D. W. and Inoue, S., “Establishment of an Optimal Decontamination Process on aNewly Designed Semi-pilot Equipment,”Nucl. Eng. Des.,229, 91 (2004).
Kim, K., Lee, H. J., Kang, D. W. and Inoue, S., “Synthesis of Simulated Cruds for Development of Decontaminating Agents,”Nucl. Eng. Des.,223, 329 (2003a).
Kim, M. H., Lee, E. K., Jun, J. H., Han, G.Y., Kong, S. J., Lee, B. K., Lee, T. J. and Yoon, K. J., “Hydrogen Production by Catalytic Decomposition of Methane over Activated Carbons: Deactivation Study,”Korean J. Chem. Eng.,20, 835 (2003b).
Kim, S. J., Shim, W.G., Kim, T. Y., Moon, H., Kim, S. J. and Cho, S. Y., “Adsorption Equilibrium Characteristics of 2,4-Dichlorophenoxyacetic Acid and 2,4-Dinitrophenol on Granular Activated Carbons,”Korean J. Chem. Eng.,19, 967 (2002).
Kong, S. H., Kown, C. I. and Kim, M. H., “Ozone Kinetics and Diesel Decomposition by Ozonation in Groundwater,”Korean J. Chem. Eng.,20, 293 (2003).
Lee, H. J., Kang, D. W., Chi, J. and Lee, D. H., “Degradation Kinetics of Recalcitrant Organic Compounds in a Decontamination Process withUV/H2O2 andUV/H2O2/TiO2 Processes,”Korean J. Chem. Eng.,20, 503 (2003).
Lee, H. J., Kang, D. W. and Lee, Y. J., “An Electrochemical Study of Stainless Steels and a Nickel Alloy in a Decontamination Agent Using the Potentiodynamic Method,”Korean J. Chem. Eng.,21, 895 (2004).
Lücking, F., Köser, H., Jank, M. and Ritter, A., “Iron Powder, Graphite and Activated Carbon As Catalysts for the Oxidation of 4-Chlorophenol with Hydrogen Peroxide in Aqueous Solution,”Water Research,32, 2607 (1998).
Moon, J. K., Byun, K. H., Park, S. Y. and Oh, W. Z., “Dynamic Simulation of Cyclic Voltammogram on the Electrochemical Reduction of VO2+ Ions,”Korean J. Chem. Eng.,14, 521 (1997).
Nowack, B. and Sigg, L., “Adsorption of ETA and Metal-EDTA Complexes onto Goethite,”J. Colloid Interf. Sci.,177, 106 (1996).
Nowack, B., Lûtzenkirchen, J., Behra, P. and Sigg, L., “Modeling the Adsorption of Metal-EDTA Complexes onto Oxides,”Environ. Sci. Technol.,30, 2397 (1996).
Ocken, H.,Decontamination Handbook, EPRI Report TR−112352, EPRI (Electric Power Research Institute), Palo Alto (1999).
Ravikmar, J. X. and Gurol, M. D., “Chemical Oxidation of Chlorinated Organics by Hydrogen Peroxide in the Presence of Sand,”Environ. Sci. Technol.,28, 394 (1994).
Ridge, A. C. and Sedlak, D. L., “Effect of Ferric Chloride Addition on the Removal of Cu and Zn Complexes with EDTA During Municipal Wastewater Treatment,”Water Research,38, 921 (2004).
Seco, A., Marzal, P., Gabaldón, C. and Ferrer, J., “Adsorption of Heavy Metals from Aqueous Solutions onto Activated Carbon in Single Cu and Ni System and in Binary Cu-Ni, Cu-Cd and Cu-Zn Systems,”J. Chem. Technol. Biotechnol.,68, 23 (1997).
Song, J. H., Yeon, K. H., Cho, J. and Moon, S. H., “Effects of the Operating Parameters on the Reverse Osmosis-Electrodeionization Performance in the Production of High Purity Water,”Korean J. Chem. Eng.,22, 108 (2005).
Toles, C. A., Marshall, W E. and Jones, M. M., “Granular Activated Carbons from Nutshells for the Uptake of Metals and Organic Compounds,”Carbon,35, 1407 (1997).
Varrin, R. Jr.,Characterization of PWR Steam Generator Deposits, EPRI Report TR−106048, EPRI, Palo Alto (1996).
Verga, K., Baradlai, P., Hirschberg, G., Németh, Z., Oravetz, D., Schunk, J. and Tilky, P., “Corrosion Behavior of Stainless Steel Surfaces Formed upon Chemical Decontamination,”Electrochemica Acta,46, 3783 (2001).
Warhurst, A. M., Fowler, G. D., McConnachie, G. L. and Pollard, S. J. T., “Pore Structure and Adsorption Characteristics of Steam Pyrolysis Carbons from Moringa Oleifera,”Carbon,35, 1039 (1997).
Wood, C. J. and Spalaris, C. N.,Sourcebook for Chemical Decontamination of Nuclear Power Plants, EPRI Special Report NP-6433, EPRI, Palo Alto (1989).
Yim, M. S. and Ocken, H., “Radiation Dose Management in Nuclear Power Plants,”Progress in Nuclear Energy,39, 31 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, HJ., Kim, K., Kang, DW. et al. Effect of different reduction methods on the efficiencies in the chemical decontamination processes. Korean J. Chem. Eng. 22, 865–872 (2005). https://doi.org/10.1007/BF02705666
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705666