Abstract
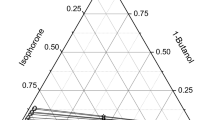
The Experimental binodal curve, tie line data, plait point data and the tie lines and plit point, calculated by liquid models for foil wing six ternary liquid liquid equilibria systems: monochlorobenzene-water-acetone, cyclohexane-water-acetone, cyclohexane-water-acetone. chlorform-water-acetone. methylisobutylketone-water-acetone, and n-hexane-water-acetone were reported at 10°C
Experimental tie line data were correlated to test consistency with Othmer-Tobias equation [15]
And those data were also correlated with the NRTL the UNIQUAC, and the modified UNIQLAC models respectively and the parameters in each model were estimated to predict the value of tie lines by least squares methods.
Similar content being viewed by others
References
Bottini S.:J. Chem. Eng. Data.,31, 84(1986)
Varhegyi, G, and Eon C.H.:I & FC Fundam.,16, 182(1977)
Anderson, T. F. and Prausmtz, J. M.:I & EC Pro. Des, Dev.,17, 561 (1978)
Tochigi, K., Hiraga, M. and Kojima, K.:J. Chem. Eng. Japan.,13, 159 (1980)
Rasmussen, P. and Fredenslund, A.:I & EC Pro. Des Dev.,20, 331 (1981)
Mukhopadhyay, M. and Sahasranaman K.:I & EC Pro. Des. Dav.,21, 632 (1982)
Perry, R. H. and Green, D.: Perry’s Chemical Engineer’s Handbook’, 6th ed., McGraw-Hill New York, NY(1984)
Fredenslund, A., Jones, R. L. and Prausmtz, J.M.:AIChE J. 21, 1086(1975)
Haddad, P. and Ednnster, W.C.:J. Chem. Eng. Data,17, 275(1972)
Choi, J.S. and Rhim J.N.:WHAHAK KONGHAK 24, 185(1986)
Othmer, D.F., White, R.E. and Tuieger, E.:lnd. Eng. Chem.,33, 1240(1941)
Radeeki, A., Kaczniaiek, B. and Grzybowski, J.:J. Chem. Eng. Data.,20, 163(1975)
Trevbal, R. E. Weber, D. and Daley, J.F.:lnd Eng. Chem.,38, 817(1946)
Hand, D.B.:J. Phys. Chem.,34, 693(1930)
Othmer, D.F. and Tobias, P.E.:Ind, Eng. Chem.,38, 87(1946)
Sorensen, J. M., Magnussen T., Rasmussen, P. and Freedenslund, A.:Fluid Phase Lquilib.,3, 47 (1979)
Abrams, D.S. and Prausmlz, J.M.:AlChE. J.,21, 116(1975)
Anderson, T.F. and Prausnitz, J.M.:I & EC Pro. Des. Dev.,17, 552 (1978)
Renon, H. and Prausnitz, J.M.:AlChE J. 14, H5 (1968)
Hooke, R. and Jeeves, T.A.:J. Assoc Comp. Mach.,8, 212 (1961)
Prausnitz, J.M., Anderson, T.F., Giens, E.A., Ekert, C. A., Hsieh, R. and O’connell J.P.: Computer Calculations for Multicomponent Vapor-Liquid and Liquid-Liquid Equilibria. Prentice-Hall, Inc., Englevvood, N. J., 145 (1980)
Goggenheim, E.A.: ‘Mixtures’, Clarendon Press Oxford (1952)
Triday, J.O.:J. Chem. Eng. Data.,29, 321 (1984)
Annesini M.C., Gnom F. and Marrelli, L.J. Chem. Eng. Data.,30, 195(1985)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Choi, J.S., Park, D.W. & Rhim, J.N. Prediction of ternary liquid-liquid equilibria using the NRTL and the UNIQUAC models. Korean J. Chem. Eng. 3, 141–151 (1986). https://doi.org/10.1007/BF02705026
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705026