Abstract
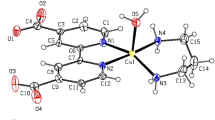
Cu(I) complexes of formula Cu(PPh3)2LClO4 [L = 2 or 3 pyridine carboxaldehyde] are synthesised and characterised to explore the coordination of an aldehyde, a hard and neutral oxygen donor to a soft Cu(I) centre. The structural and spectroscopic results illustrate that only in 2-pyridinecarboxaldehyde, the ‘C=O’ group coordinates to soft Cu(I) centres due to a favourable chelate effect, while in 3-pyridinecarboxaldehyde, it remains uncoordinated. Upon chelation via N and O donors, 2-pyridinecarboxaldehyde resembles bipyridine or phenanthroline in terms of its bite angle and spectroscopic features. Such chelation can be easily challenged with coordinating anions like bromide, or more basic pyridines. A drastic change in the MLCT absorption signals the decomplexation of the ‘C=O’ group. The observed results point out that the Cu(I) centre can readily exchange the hard ‘O’ donor for softer ligands.
Similar content being viewed by others
References
Hathaway B J 1987 InComprehensive coordination chemistry (eds) G Wilkinson, R D Gillard and J A McCleverty (Oxford: Pergamon) vol. 5, p. 534
Pearson R G 1968J. Chem. Edu. 45 581, 643
Cotton F A and Wilkinson G 1988Advanced inorganic chemistry 5th edn (New York: Wiley Interscience)
Churchill M R, De Boer B D, Rotella F J, Abu Salah O M and Bruce M I 1975Inorg. Chem. 14 2051;
Pasquali M, Floriani C and Gaetani-Manfredotti A 1980Inorg. Chem.19 1191;
Pasquali M, Marchetti F and Floriani C 1978Inorg. Chem. 17 1684;
Pasquali M, Floriani C, Venturi G, Gaetani-Manfredotti A and Chesi-Villa 1982J. Am. Chem. Soc. 104 4092
Saravanabharati D, Monica, Venugopalan P and Samuelson A G 2002Polyhedron (in press);
Saravanabharathi D, Nethaji M and Samuelson A G 2002Polyhedron (submitted)
Dyason J C, Engelhardt L M, Healy P C, Klich H L and White A W 1986Aust. J. Chem. 39 2003;
Hart R D, Healy P C, Hope G A, Turner D W and White A H 1994J. Chem. Soc.,Dalton Trans. 773;
Gaughan A P, Dori Z and Ibers J A 1974Inorg. Chem. 13 1657;
Messmer G G and Palenik G J 1969Inorg. Chem. 8 2750;
Restivo R J, Costin A, Ferguson G, Carty A 1975Can. J. Chem. 53 1949;
Darensbourg D J, Holtcamp M W, Khandelwal B and Reibenspies J H 1995Inorg. Chem. 34 5390;
Lopes C, HåKansson M and Jagner S 1997Inorg. Chim. Acta 254 361;
Jung B, Karlin K D and Zuberbühler A D 1996J. Am. Chem. Soc. 118 3763
Pilloni G, Corain B, Degano M, Longato B and Zanotti G 1993J. Chem. Soc., DaltonTrans. 1777
Perrin D D and Armarego W L FPurification of laboratory chemicals 3rd edn (London: Pergamon)
WinGX (1.63.02), An integrated system of Windows program for the solution, refinement, and analysis of single crystal X-ray diffraction
Walker N and Start D 1983Acta Crystallogr. A39 158
Sheldrick G M 1986 SHELXS86, Program for crystal structure determination; University of Cambridge, England; Sheldrick G M 1997 SHELXL97, Program for crystal structure refinement; University of Göttingen, Göttingen, Germany
Munakata M, Wu L P, Kuroda-Sowa T, Maekawa M, Moriwaki, K and Kitawaga S 1997Inorg. Chem. 36 5416;
Munakata M, Kuroda-Sowa T, Maekawa M, Nakamura M, Akiyama S and Kitawaga S 1994Inorg. Chem. 33 1284;
Wu L P, Yamamoto M, Kuroda-Sowa T, Maekawa M, Suenaga, Y and Munakata M 1996J. Chem. Soc., Dalton Trans. 2031
Munakata M, Kitawaga S and Emori T 1991J. Chem. Soc., Chem. Commun. 2031;
Lin S, Lin C J, Cheng S T, Wen Y S and Liu L K 1997Inorg. Chim. Acta 256 35
Goher MAS, Abdou A E H, Wai-Hing Yip and Mak T C W 1993Polyhedron 2981
Engelhardt L M, Pakawatchai C and White A H 1985J. Chem. Soc., Dalton Trans. 125
Kitagawa S, Maruyama H, Wada S, Munakata M, Nakamura M and Masuda H 1991Bull. Chem. Soc. Jpn. 64 2809
Coffey S 1976Rodd's Chemistry of carbon compounds 2nd edn (Amsterdam: Elsevier Scientific) vol. 4
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saravanabharathi, D., Nethaji, M. & Samuelson, A.G. Is copper(I) really soft? Probing the hardness of Cu(I) with pyridinecarboxaldehyde ligands. J Chem Sci 114, 347–356 (2002). https://doi.org/10.1007/BF02703825
Issue Date:
DOI: https://doi.org/10.1007/BF02703825