Abstract
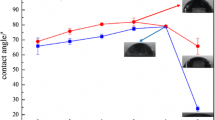
Resolve-type phenolic polymer coatings were deposited on carbon steel panels, and then were exposed for 15 days to a simulated geothermal brine with pH 1.6 at 150°, 175°, and 200°C. The phenolic polymers were hydrothermally oxidized. To comprehensively understand the mechanisms of this oxidative degradation of the coatings, the modern analytical techniques of XPS, contact angle, TGA, SEM-EDX, and EIS were used in combination. The oxidative degradation of polymer took place in the three-step oxidation routes: first, the bridging methylene linkages in the network polymer structure were replaced by the benzlhydrol-type linkages; second, the benzlhydrol-type linkages were transformed into the benzophenone-type linkages; and finally, the C-C-C linkage in the benzophenone derivative ruptured to form salicylic acid derivatives as the ultimate degradation products. Hydrothermal temperatures of >175°C promoted the degree of such oxidative degradation, causing the coating surface to become susceptible to moisture, to absorb more brine, and also to allow corrosive electrolytes to permeate easily. Consequently, iron oxides as the corrosion products from the underlying steel were yielded at a critical interfacial zone between the coating and steel. The excessive growth of iron oxides led to the generation of internal stress-induced cracks in the coating film, thereby resulting in the failure of these corrosion-preventing barriers.
Similar content being viewed by others
References
Sugama, T., “Interfaces Between Geothermal Brine-Induced Scales and SiC-Filled Polymer Linings,”Geothermics, 27, 387 (1998).
Sugama, T., Webster, R., Reams, W., and Gawlik, K., “High-Performance Polymer Coatings for Carbon Steel Heat Exchanger Tubes in Geothermal Environments,”J. Mater. Sci., 35, 2145 (2000).
Sugama, T., “Polyphenelenesulfide-sealed Ni-Al Coatings for Protecting Steel from Corrosion and Oxidation in Geothermal Environments,”J. Mater. Sci., 33, 3791 (1998).
Ulrich, H.,Introduction to Industrial Polymers, Hanser Publishers, New York, pp 141–143, 1993.
Era, V.A. and Mattila, A., “Thermal Analysis of Thermosetting Resins,”J. Therm. Anal., 10, 461 (1976).
Fridman, H.L., “Kinetics of Thermal Degradation of Charforming Plastics from Thermogravimetry: Application to a Phenolic Plastic,”J. Polym. Sci. Part C, 6, 183 (1964).
Alaminov, H. and Andonova, N., “Thermal Decomposition of Phenol Formaldehyde Resins Modified with Cyanuric Acid,”J. Appl. Polym. Sci., 14, 1083 (1970).
Clark, D.T. and Thomas, H.R., “Applications of ESCA to Polymer Chemistry. X. Core and Valence Energy Levels of a Series of Polyacrylates,”J. Polym. Sci., 14, 1671 (1976).
Kelly, M.A., “More-powerful ESCA Makes Solving Surface Problems a Lot Easier,”Research & Development, January, 80 (1984).
Scantlebury, J.D., and Sussex, G.A.,Corrosion Control by Organic Coatings, Leidheiser, H. Jr. (Ed.), National Association of Corrosion Engineers, Houston, pp 51–55, 1981.
Mansfield, F., Kendig, M.W., and Tsai, S., “Evaluation of Corrosion Behavior of Coated Metals with AC Impedance Measurements,”Corrosion, 38, 478 (1982).
Gerenser, L.J.,Metallization of Polymers, Pireaux, J. and Kowalczyk, S.P. (Eds.), ACS Symposium Series 440, American Chemical Society, pp 433–452, Washington D.C., 1990.
Katovic, Z., “Curing of Resole-Type Phenol-Formaldehyde Resin,”J. Appl. Polym. Sci., 11, 85 (1967).
Briggs, D., Brewis, D.M., and Konieczko, M.B., “X-ray Photoelectron Spectroscopic Studies of Polymer Surfaces,”J. Mater. Sci., 14, 1344 (1979).
Briggs, D. and Seah, M.P.,Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy, John Wiley & Sons, Ltd., New York, pp 362–364, 1983.
Jackson, W.M. and Conley, R.T., “High Temperature Oxidative Degradation of Phenol-Formaldehyde Polycondensate,”J. Appl. Polym. Sci., 8, 2163 (1964).
Conley, R.T., “Oxidative Degradation of Phenol-Formaldehyde Polycondensation Initial Degradation Reactions,”J. Appl. Polym. Sci., 9, 1117 (1965).
Author information
Authors and Affiliations
Additional information
This program report, issued by Raymond LaSala (Program Manager, DOE Office of Geothermal and Wind Technologies), was performed under the auspices of the U.S. Department of Energy, Washington, D.C. under Contract No. DE-AC02-98-CH10866.
Materials and Chemical Sciences Div., Dept. of Applied Science, Upton, NY 11973.
1617 Cole Blvd., Golden, CO 80401.
Rights and permissions
About this article
Cite this article
Sugama, T., Kelley, S.S. & Gawlik, K. Hydrothermal degradation study of phenolic polymer coatings by advanced analytical methods. Journal of Coatings Technology 73, 65–71 (2001). https://doi.org/10.1007/BF02698399
Issue Date:
DOI: https://doi.org/10.1007/BF02698399