Abstract
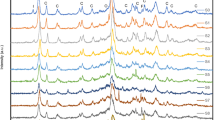
A clinoptilolite-type natural zeolite was pretreated by HC1, NaOH, and NaCl solutions to improve the ion-exchange capacities for heavy metal ions such as copper, lead, cadmium, and cesium. The pretreated natural zeolite was experimentally investigated based on chemical analyses, X-ray diffraction, and BET experiments etc. From experimental data, it was shown that the pretreatment with NaCl gave the best ion-exchange capacity for all metal ions encountered. The ion-exchange capacity of the NaCl-treated sample is comparable with that of a commercialized natural zeolite, chabazite. On the other hand, the HCl-treated sample has very low ion-exchange capacities for all metal ions even though it has high specific surface area and total pore volume. It was proven by chemical analyses that a strong acid like HC1 can damage the structure of the zeolite by extracting aluminum and iron from their skeletal units.
Similar content being viewed by others
References
Colella, C., “Ion Exchange Equilibria in Zeolite Minerals,”Mineral. Deposita,31, 554 (1996).
Kim, Y., Kim, D. S., Jang, S. B. and Park, S. Y., “Studies on the Removal of Metal Ions with Domestic Pohang Zeolites and Synthetic Zeolites,”J. KSEE,18(5), 587 (1996).
Lee, H. G., “Adsorption of Aqueous Radioactive Cs and Sr onto Zeolites,” PhD thesis, Dept. of Ind. & Eng. Chemistry, Chonnam National University (1997).
Lowell, S. and Shields, J. E., “Powder Surface Area and Porosity,” Chapman and Hall, London (1984).
Pansini, M., “Natural Zeolites as Cation Exchangers for Environmental Protection,”Mineral. Deposita,31, 563 (1996).
Perona, J. J., “Model for Sr-Cs-Ca-Mg-Na Ion-Exchange Equilibria on Chabazite,”J. AIChE,39(10), 1716 (1993).
Pradas, E. G., Sanchez, M. V., Cruz, F. C., Viciana, M. S. and Pérez, M. F., “Adsorption of Cadimium and Zinc from Aqueous Solution on Natural and Activated Bentonite,”J. Chem. Tech. Biotechnol,59, 289 (1994).
Sumner, M. E. and Miller, W. P., “Cation Exchange Capacity and Exchange Coefficients. In: Sparks, D. L. (ed.) Methods of Soil Analysis. Part 2 : Chemical Properties,” (3rd ed.) ASA, SSSA, CSSA, Madison, WI (1979).
Sun, Y S. and Kim, P. K., “Adsorption Characteristics of Cu(II) in the Presence of Surfactants on Natural Zeolites Treated Chemically and Thermally,”J. Korean Ind. & Eng. Chemistry,7(5), 849 (1996)
Zamzow, M. J., Eichbaum, B. R., Sandgren, K. R. and Shanks, D. E., “Removal of Heavy Metals and Other Cations from Wastewater using Zeolites,”Sep. Sci. & Tech.,25(13-15), 1555 (1990).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, D.H., Kim, S.J. & Moon, H. Preparation of a clinoptilolite-type korean natural zeolite. Korean J. Chem. Eng. 16, 525–531 (1999). https://doi.org/10.1007/BF02698279
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02698279