Abstract
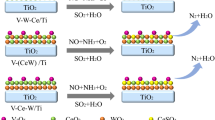
As an attempt to improve the catalytic activity at higher reaction temperatures between 300-450°C, various mole ratios of WO3 were added to V2O5/TiO2 catalytic systems. And also, in order to suggest a new mixed oxide catalyst system for simultaneous removal of NOx and SOx, from stationary sources, MoO3-V2O5/TiO2catalysts were prepared by a conventional impregnation method together with a newly introduced method of surface fixation (non-aqueous solution method). In case of WO3 addition, at higher reaction temperature range (300–450°C), WO3 and WO3-V2O5/TiO2 catalysts showed significant high conversion in NO reduction with NH3 while V2O5/TiO2 catalyst showed a significant change in selectivity mainly due to the excess side reaction of NH3 oxidation. This difference in selectivity due to NH3 oxidation at high temperature is supposed to be associated with the difference in values of surface excess oxygen between WO3 and V2O5 on titania. The surface acidities of tested catalysts were relatively well correlated with the % conversion of NO at 400°C. In case of MoO3 addition, the catalytic activity for the simultaneous removal of NOx and SOx were quite enhanced by the addition of MoO3 into V2O5/TiO2 catalysts. The enhanced activities were responsible for the formation of Mo=O bond on the intermediate species produced by solid solutions on MoO3-V2O5/TiO2 (aqueous). However, in the case of MoO3-V2O5/TiO2 (non-aqueous), the exact source of active site was not able to detect in IR spectra in spite of more enhanced activity was obtained in this study. After SO2 contact, VOSO4 is newly formed on the surface of catalyst, which supposed to be associated with the activity enhancement.
Similar content being viewed by others
References
Teraoka, Y. and Yamazoe, A.:Shokubai,29(6), 530 (1987).
Kato, A., Matsud, S. and Kamo, T.:Ind. Eng. Chem. Prod. Res. Dev.,22, 406 (1983).
Lee, J. B., Gu, M. H. and Choung, S. J.: Proceedings of the 8th ROC/ROK Joint Symposium on Catalysis, October 1–2, Taiwan, Republic of China, p. 39 (19 91).
Tanabe, K.: “Solid Acids and Bases”, Academic Press, p. 17 (1970).
Morikawa, S., Takahashi, K., Yoshida, H. and Kurita, S.:8th Int. Cong, on Catalysis,3, 661 (1984).
Satsuma, A., Hattori, A., Mizutami, K., Furuka, A. and Miyamoto, A.:J. Phys. Chem.. 93, 1484 (1989).
Tarama, K., Teranishi, S., Yoshida, S. and Tamura, N.:Prod. Int. Cong. Catal,3, 282 (1965).
Tarama, K., Yoshida, S., Ishida, S. and Kakioka, H.:Bull. Chem. Soc. Japan,41, 2840 (1968).
Teraoka, A., Yamashida, A. and Yamazoe, N.:Chem. Lett., p. 1301 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shin, BS., Lim, SY. & Choung, SJ. WO3 and MoO3 addition effect on V2O5/TiO2 as promoters for removal of NOx and SOx from stationary sources. Korean J. Chem. Eng. 11, 254–260 (1994). https://doi.org/10.1007/BF02697392
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02697392