Abstract
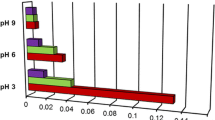
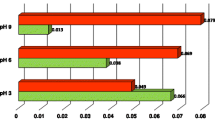
The effect of adsorbed polymer on the stability of alumina suspension was investigated. Poly(ethylene oxide) (PEO), poly(acrylic acid) (PAA) and similar kinds of polymer salts were used as a dispersant. The amount of polymer adsorbed on alumina surface and the suspension stability was measured. The pH, molecular weight, and concentration were considered as experimental parameters. PEO shows low affinity on the alumina surface while PAA has high affinity. In the case of PAA adsorption, the surface charge change by polymer adsorption influences suspension stability strongly, but not in the case of PEO adsorption. In simultaneous adsorption of PEO and PAA, the PAA concentration was fixed and PEO concentration was varied. The stability of suspension increased with increasing PEO concentration, and this is partly due to the steric stabilization by adsorption of PAA-PEO complex or adsorption of PEO through pre-adsorbed PAA and the depletion effect of non-adsorbed polymer. Suspension adsorbing sodium salts of PAA and poly(methacrylic acid) (PMA) each showed similar stability. But, when the PEO and these kinds of salts were added together to the suspension, the one with PAA sodium salt could keep a higher stability even with lower molecular weights of PEO compared with suspension with PMA sodium salt.
Similar content being viewed by others
References
Chi-Jen, S. and Min-Hsiung, H., “Stabilization of Aqueous Si3N4 Sus-pensions with Ammonium Salt of Poly(acrylic acid) at Various pH”,Mater. Chem. and Phys.,57, 125 (1998).
Esumi, K., Ishuzuki, K., Otsuka, H., Ono, M., Ichikawa, S. and Yanase, C. J., “The Effect of Binary Solvents on Adsorption of Poly(vinylpyr-rolidone) on Titanium Dioxide and Graphite Particles”,J. Colloid Interface Sci.,178, 549 (1996).
Fendler, J. H., “Colloidal Chemical Approach to NanotechnologieKorean J. Chem. Eng.,18, 1 (2001).
Hirata, Y., Kamikakimoto, J., Nishimoto, A. and Mrihara, Y, “Interaction between Alpha-alumina Surface and Polyacrylic Acid”,J. Ceram. Soc. Japan,100, 7 (1992).
Ishiduki, K. and Esumi, K., “Adsorption Characteristics of Poly (acrylic acid) and Poly(vinyl pyrollidone) on Alumina from Their Mixtures in Aqueous Solutions”,J. Colloid Interface Sci.,185, 274 (1997).
Kawaguchi, M., Kawaguchi, H. and Takahashi, A., “Competitive and Displacement Adsorption of Polyelectrolyte and Water-Soluble Nonionic Polymer at the Silica Surface”,J. Colloid Interface Sci.,124, 57 (1987).
Mathur, S. and Moudgil, B. M., “Adsorption Mechanism(s) of Poly (ethylene oxide) on Oxide Surfaces”,J. Colloid Interface Sci.,196, 92 (1997).
Santhiya, D., Subramanian, S., Natarajan, K. A. and Malghan, S. G., “Surface Chemical Studies on the Competitive Adsorption of Poly (acrylic acid) and Poly(vinyl alcohol) onto Alumina”,J. Colloid Inter-face Sci.,216, 143 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeon, S.J., Hong, W.H. Effects of poly(acrylic acid) and poly(ethylene oxide) adsorption on the stability of alumina suspension. Korean J. Chem. Eng. 20, 916–921 (2003). https://doi.org/10.1007/BF02697299
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02697299