Abstract
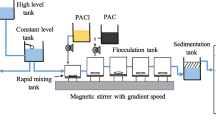
The objectives of this research are to identify the membrane fouling potential due to different fractions of NOM and correlate the physicochemical properties of NOM and membranes with the adsorption of humic substances on membrane and investigate the mechanism of coagulation affecting UF, and find the optimum conditions of the combined of coagulation with UF membrane filtration for NOM removal. For Nakdong river water, the humic acid fraction was the most reactive precursor fraction for the formation of the ratio of THMFP/DOC (STHMFP) and TOXFP/DOC (STOXFP). The result of adsorption kinetics tests showed that hydrophobic organics adsorbed much more quickly than hydrophilic organics on both membranes. Thus, hydrophobic compounds exhibited a preferential adsorption onto membrane. In case of the effect of membrane properties on the adsorption of organic fractions, the adsorption ratio (C1/Ce) was greater for the hydrophobic membrane than for the hydrophilic membrane regardless of the kind of organic fractions. For combined coagulation with membrane process, flux reduction rate showed lower than the UF process alone. Also, the rate of flux decline for the hydrophobic membrane was considerably greater than for the hydrophilic membrane. Applying the coagulation process before membrane filtration showed not only reduced membrane fouling, but also improved removal of dissolved organic materials that might otherwise not be removed by the membrane. That is, during the mixing period, substantial changes in particle size distribution occurred under rapid and slow mixing conditions due to the simultaneous formation of microflocs and NOM precipitates. Therefore, combined pretreatment using coagulation (both rapid mixing and slow mixing) improved not only dissolved organic removal efficiency but also DBP (Disinfection By-Product) precursor's removal efficiency.
Similar content being viewed by others
Abbreviations
- DBPFP:
-
Disinfection By Product Formation Potential
- STHMFP:
-
Specific Trihalomethane Formation Potential (Μg THMFP/mg DOC)
- STOXFP:
-
Specific Total Organic Halogen Formation Potential (ΜgTOXFP/mgDOC)
- MWCO:
-
Molecular Weight Cut Off
- AMW:
-
Apparent Molecular Weight
- SUVA:
-
Specific UV Absorbance (UV254/DOC)
- C(t):
-
the amount of organics adsorbed at time [t]
- C(e):
-
the amount which can adsorb on the membrane surface at equilibrium
- J0 :
-
permeate flux of initial purewater
- Ji :
-
permeate flux at each time [t]
- G:
-
velocity gradient
- UFC:
-
Uniform Formation Condition
References
Agbekodo, M. K., Legube, B. and Cote, P., “Organics in NF Permeate”,J. Am. Water Works. Assoc.,88(5), 67 (1996).
Amicon Inc., Laboratory separation, Product catalog, publication, 716 (1987).
APHA-AWWA-WEF, “Standard Methods for the Examination of Water and Wastewater”, APHA-AWWA-WEF, 20th eds. (1998).
Cheryan, M., “Ultrafiltration Handbook”, Technomic Publishers, Lancaster, PA (1986).
Collins, M., Amy, G. and Steelink, C, “Molecular Weight Distribution, Carboxylic Acidity, and Humic Substances Content of Aquatic Organic Matter: Implications for Removal During Water Treatment”Environ. Sci. Technol.,20(10), 1028 (1986).
Croue, J. P., Martin, B., Deguin, A. and Legube, B., “Isolation and Characterization of Natural Organic Matter from Surface Water/Comparison of Resin Adsorption and Membrane Filtration Isolation Procedure”, Natural Organic Matter Workshop, Poitiers, France, September '96, 6 (1996).
Cuperus, F. P. and Smolders, C. A., “Characterization of Ultrafiltration Membranes: Membrane Characteristics and Characterization Techniques”,Adv. Colloid Interface Sci.,35, 135 (1991).
Fu, P., Ruiz, H., Thompson, K. and Spangenberg, C., “Selecting Membranes for Removing NOM and DBP Precursors”,J. Am. Water Works. Assoc.,86(12), 55 (1994).
Hermia, J., “Constant Pressure Blocking Filtration Laws: Application to Power-law Non-Newtonian Fluids”,Trans. Inst. Chem. Eng.,60, 183 (1982).
Jacangelo, J. G., Laine, J. M., Cummings, E. W. and Adham, S. S., “UF with Pretreatment for Removing DBP Precursors”,J. Am. Water Works. Assoc.,87(3), 100 (1995).
Jung, C. W, Han, S. W. and Kang, L. S., “Characteristic of Organic Substances Adsorption onto Membrane Materials”,J. Kor. Soc. Environ. Eng.,24(n8), 1339 (2002).
Jung, C. W., “Removal of Natural Organic Matter by Combined Coagulation-UF Membrane Process for Water Treatment/rd Ph. D., thesis, Department of Environmental Engineering, Pukyong National University, Busan (2002).
Kang, L. S., Han, S. W. and Jung, C. W., “Synthesis and Characterization of Polymeric Inorganic Coagulants for Water Treatment”,Korean J. Chem. Eng.,18(6), 965 (2001).
Kim, E. J., Jung, C W, Choi, S. H. and Kang, L. S., “The Influence of Rapid Mixing Condition on Coagulation in Water Treatment”J. Kor. Soc. Environ. Eng.,23(4), 631 (2001).
Laine, J. M., Clark, M. M. and Mallevialle, J., “Ultrafiltration of Lake Water: Effects of Pretreatment on the Partitioning of Organics, THMFP, and Flux”,J. Am. Water Works. Assoc,82(12), 82 (1990).
Laine, J. M., Hagstrom, J. P., Clark, M. M. and Mallevialle, J., “Effects of UF Membrane Composition”,J. Am. Water Works. Assoc.,81(11), 61 (1989).
Lahoussine-Turcaud, V, Wiesner, M. R. and Bottero, J. Y., “Fouling in Tangential-flow Ultrafiltration: the Effect of Colloid Size and Coagulation Pretreatment”,J. Membrane. Sci.,52(2), 173 (1990).
Lee, C. K. and Hong, J., “Characterization of Electric Charges in Microporous Membranes”,J. Membrane. Sci.,39(1), 79 (1988).
Logan, B. E. and Jiang, Q., “Molecular Size Distribution of Dissolved Organic Matter”,J. Env. Engrg.,116(6), 1046 (1990).
McCabe, W L., Smith, J. C. and Harriott, P., “Unit Operations of Chemical Engineering” McGraw-Hill, New York, USA (1985).
Summer, R S., Hooper, S. M., Shukairy, H. M., Solaril, G. and Owen, D., “Assessing DBP Yield: Uniform Formation Conditions”,J. Am. Water Works. Assoc,88(6), 80 (1996).
Thurman, E. and Malcolm, R., “Preparative Isolation of Aquatic Humic Substances”,Environ. Sci Technol.,15(4), 463 (1981).
Tipping, E. and Ohnstad, M., “Aggregation of Aquatic Humic Substances”,Chem. Geol.,44, 349 (1984).
Wiesner, M. R., Lahoussine-Turcaud, V. and Fiessinger, F, “Organic Removal and Particle Formation using a Partially Neutralized A1C13”, In Proceedings of the Annual Conference of the American Water Works Association, Denver, CO (1986).
Yuan, W. and Zydney, A. L., “Humic Acid Fouling During Microfiltration”J. Membrane Sci.,157(1), 1 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, CW., Kang, LS. Application of combined coagulation-ultrafiltration membrane process for water treatment. Korean J. Chem. Eng. 20, 855–861 (2003). https://doi.org/10.1007/BF02697288
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02697288