Abstract
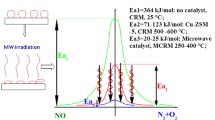
Effects of microwaves on catalytic reaction systems are analyzed theoretically in this work. Use of microwaves is encouraged to save energy. The effects of microwave heating are analyzed theoretically by assuming that the catalyst pellet is homogeneous. The temperature and concentration profiles within the catalyst pellet were obtained by numerical simulations for the cases of microwave heating and conventional heating. In the modeling the catalyst pellet is regarded as a continuum. When a chemical reaction was conducted in a heterogeneous medium with microwave heating, the reaction rate and the yield were found to be increased compared to conventional heating under the same reaction conditions. This is due to hot spots generated by selective heating of the catalyst pellet, resulting in an increased reaction rate.
Similar content being viewed by others
References
Adu, B. and Otten, L., “Modeling Microwave Heating Characteristics of Granular Hygroscopic Solids,”J. Microwave Power and Electromagn. Energy,31, 35 (1996).
Berlan, J., Giboreau, P., Lefeuvre, S. and Marchand, C., “Synthese Organique Sous Champ Microondes: Premier Exemple d'Activation Specifique en Milieu Homogene,”Tetrahedron Lett.,32, 2363 (1991).
Berlan, J., “Microwaves in Chemistry: Another Way of Heating Reacting Mixtures,”Radiat. Phys. Chem.,45, 581 (1995).
Chemat, F., Esveld, D. C., Poux, M. and Di-Martino, J. L., “The Role of Selective Heating in the Microwave Activation of Heterogeneous Catalysis Reactions Using a Continuous Microwave Reactor,”J. Microwave Power and Electromagn. Energy,33, 87 (1998).
Datta, A.K. and Liu, J., “Thermal Time Distributions for Microwave and Conventional Heating of Food,”Trans. IChemE.,70, 1327 (1992).
Hill, J.M. and Marchant, T. R., “Modeling Microwave Heating,”Appl. Math. Modelling,20, 3 (1994).
Jahngen, E.G. E., Lentz, R.R., Pesheck, P. S. and Sackett, P. H., “Hydrolysis of Adenosine Triphosphate by Conventional or Microwave Heating,”J. Org. Chem.,55, 3406 (1990).
Le Bail, A., Koutchma, T. and Ramaswamy, H. S., “Modeling of Temperature Profiles Under Continuous Tube-Flow Microwave and Steam Heating Conditions,”J. Food Process Eng.,23, 1 (1999).
Metaxas, A. S. and Meredith, R. J., “Industrial Microwave Heating,” IEE Power Engineering Series 4, London: P. Peregrinus (1983).
Mingos, D. M. P. and Baghurst, D. R., “Applications of Microwave Dielectric Heating Effects to Synthetic Problems in Chemistry,”Chem. Soc. Rev.,20, 1 (1991).
Park, H. C. and Moon, H., “A Numerical Analysis of Rate Data for Packed Bed Reactors in Gas-Solid Reaction Systems,”Korean J. Chem. Eng.,4, 135 (1987).
Pipus, G., Plazl, I. and Koloini, T., “Esterification of Benzoic Acid in Microwave Tubular Flow Reactor,”Chem. Eng. J.,76, 239 (2000).
Plazl, I., Pipus, G. and Koloini, T., “Microwave Heating of the Continuous Flow Catalytic Reactor in a Nonuniform Electric Field,”AIChE J.,43, 754 (1997).
Pollington, S.D., Bond, G., Moyes, R. B., Whan, D. A., Candlin, J. P. and Jennings, J. R., “The Influence of Microwaves on the Rate of Reaction of Propan-1-ol with Ethanoic Acid,”J. Org. Chem.,56, 1313 (1991).
Raner, K. D. and Strauss, C. R., “Influence of Microwaves on the Rate of Esterification of 2,4,6-Trimethylbenzoic Acid with 2-Propanol,”J. Org.Chem.,57, 6231 (1992).
Sun, W.C., Guy, P.M., Jahngen, J. H., Rossomando, E. F. and Jahngen, E. G. E., “Microwave-Induced Hydrolysis of Phospho Anhydride Bonds in Nucleotide Triphosphates,”J. Org. Chem.,53, 4414 (1988).
Thomas, J. M. and Thomas, W. J., “Principles and Practice of Heterogeneous Catalysis,” Weinheim, VCH (1997).
Toma, S., “Use of Microwave Radiation in Organic Synthesis,”Chem. Listy.,87, 627 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeo, YK., Kim, HY., Kim, IW. et al. Analysis of catalytic reaction systems under microwaves to save energy. Korean J. Chem. Eng. 20, 1–7 (2003). https://doi.org/10.1007/BF02697176
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02697176