Abstract
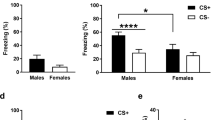
Many studies have alluded to sexually dimorphic changes in behavior following stress. Although many have suggested that these changes are a function of stress-induced changes in learning and memory, there are questions regarding whether performance in those learning and memory tasks are influenced by stress-induced changes in drive more than in actual learning and memory processes. We used the classically conditioned eyeblink response (CCER) to determine whether slowed learning following stress in females can be explained by changes in unconditional response (UR) amplitude, a sign of a stress-induced shift in sensory reactivity. In addition, we had a second treatment group injected with the pro-inflammatory cytokine IL-1β to serve as an interoceptive stress condition, a physiological stressor with minimal stimulation to the animal. Replicating the work by Shors and colleagues, we found that stressed female rats had slower acquisition of the conditioned response (CR), but we also found that an IL-1β injection leads to a slowing of CR acquisition. However, in both cases, UR amplitude was lower in the treatment groups. We followed up these results by testing sensory reactivity through the acoustic startle response (ASR), where the magnitude of the ASR was marginally, but nonsignificantly, reduced by the same dose regimen of IL-1β. Together, these experiments suggest that tailshock stress and immune signaling (IL-1β) reduce sensory reactivity and the saliency of the stimuli used in the CCER, leading to slower learning in female rats.
Similar content being viewed by others
References
Avitsur, R., Weidenfeld, J., & Yirmiya, R. (1999). Cytokines inhibit sexual behavior in female rats: II. Prostaglandins mediate the suppressive effects of interleukin-1beta.Brain Behav. Immun., 13, 33–45.
Avitsur, R. & Yirmiya, R. (1999a). Cytokines inhibit sexual behavior in female rats: I. Synergistic effects of tumor necrosis factor alpha and interleukin-1.Brain Behav. Immun., 13, 14–32.
Avitsur, R. & Yirmiya, R. (1999b). The immunobiology of sexual behavior: gender differences in the suppression of sexual activity during illness.Pharmacol. Biochem. Behav., 64, 787–796.
Beck, K. D., Brennan, F. X., & Servatius, R. J. (2002). Effects of stress on nonassociative learning processes in male and female rats.Integative. Physiol. Physiological and Behavioral Science, 37, 128–139.
Beck, K. D. & Luine, V. N. (1999). Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids.Brain Research, 830, 56–71.
Beck, K. D. & Luine, V. N. (2002). Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions.Physiological, Behavior., 75, 661–673.
Beylin, A. V. & Shors, T. J. (2003). Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience.Hormonal Behavior, 43, 124–131.
Bouman, A., Moes, H., Heineman, M. J., de Leij, L. F., & Faas, M. M. (2001a). Cytokine production by natural killer lymphocytes in follicular and luteal phase of the, ovarian cycle in humans.American Journal of Reproductive Immunology 45, 130–134.
Bouman, A., Moes, H., Heineman, M. J., de Leij, L. F., & Faas, M. M. (2001b). The immune response during the luteal phase of the ovarian cycle: increasing sensitivity of human monocytes to endotoxin.Fertil. Steril., 76, 555–559.
Bowman, R. E., Beck, K. D., & Luine, V. N. (2003). Chronic stress effects on memory: sex differences in performance and monoaminergic activity.Hormonal Behavior, 43, 48–59.
Bowman, R. E., Zrull, M. C. & Luine, V. N. (2001). Chronic restraint stress enhances radial arm maze performance in female rats.Brain Research, 904, 279–289.
Burgess, L. H. & Handa, R. J. (1992). Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats.Endocrinology, 131, 1261–1269.
Cannon, J. G. & Dinarello, C. A. (1985). Increased plasma interleukin-1 activity in women after ovulation.Science, 227, 1247–1249.
Galea, L. A., McEwen, B. S., Tanapat, P., Deak, T., Spencer, R. L., & Dhabhar, F. S. (1997). Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress,Neuroscience, 81, 689–697.
Gould, E., Beylin, A., Tanapat, P., Reeves, A., & Shors, T. J. (1999). Learning enhances adult neurogenesis in the hippocampal formation.Natural Neuroscience, 2, 260–265.
Johnson, J. D., O’Connor, K. A., Deak, T., Stark, M., Watkins, L. R., & Maier, S. F. (2002). Prior stressor exposure sensitizes LPS-induced cytokine production.Brain Behavior Immunology, 16, 461–476.
Leuner, B., Falduto, J., & Shors, T. J. (2003). Associative memory formation increases the observation of dendritic spines in the hippocampus.Journal of Neuroscience, 23, 659–665.
Luine, V., Villegas, M., Martinez, C., & McEwen, B. S. (1994). Repeated stress causes reversible impairments of spatial memory performance.Brain Research, 639 167–170.
Martin, J. T. (2000). Sexual dimorphism in immune function: the role of prenatal exposure to androgens and estrogens.European Journal of Pharmacology, 405, 251–261.
McEwen, B. S. (2000). The neurobiology of stress: from serendipity to clinical relevance.Brain Research., 886, 172–189.
McEwen, B. S. & Woolley, C. S. (1994). Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain.Experimental Gerontology, 29 431–436.
Mouihate, A., Chen, X., & Pittman, Q. J. (1998). Interleukin-1beta fever in rats: gender difference and estrous cycle influence.American Journal of Physiology, 275, R1450-R1454.
Patchev, V. K. & Almeida, O. F. (1996). Gonadal steroids exert facilitating and “buffering” effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain.Journal of Neuroscience., 16, 7077–7084.
Patchev, V. K., Hassan, A. H., Holsboer, D. F., & Almeida, O. F. (1996). The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus.Neuropsychopharmacology, 15, 533–540.
Polan, M. L., Daniele, A., & Kuo, A. (1988). Gonadal steroids modulate human monocyte interleukin-1 (IL-1) activity.Fertil. Steril., 49, 964–968.
Pugh, C. R., Fleshner, M., Watkins, L. R., Maier, S. F., & Rudy, J. W. (2001). The immune system and memory consolidation: a role for the cytokine IL-1βeta.Neurosci. Biobehav. Rev., 25, 29–41.
Servatius, R. J. (2000). Eyeblink conditioning in the freely moving rat: square-wave stimulation as the unconditioned stimulus.Journal of Meuroscientific Methods, 102, 35–42.
Servatius, R. J. & Shors, T. J. (1994). Exposure to inescapable stress persistently facilitates associative and nonassociative learning in rats.Behavioral Neuroscience, 108, 1101–1106.
Servatius, R. J. & Shors, T. J. (1996). Early acquisition, but not retention, of the classically conditioned eyeblink response is N-methyl-D-aspartate (NMDA) receptor dependent.Behavioral Neuroscience 110, 1040–1048.
Servatius, R. J., Brennan, F. X., Beck, K. D., Beldowicz, D., & Coyle-DiNorcia, K. (2001). Stress facilitates acquisition of the classically conditioned eyeblink response at both long and short interstimulus intervals.Learning & Motivation, 32, 178–192.
Shors, T. J. (2001). Acute stress rapidly and persistently enhances memory formation in the male rat.Neurobiol. Learn. Mem., 75, 10–29.
Shors, T. J., Beylin, A. V., Wood, G. E., & Gould, E. (2000). The modulation of Pavlovian memory.Behav. Brain Research., 110, 39–52.
Shors, T. J., Chua, C., & Falduto, J. (2001). Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus.Journal of Neuroscience, 21, 6292–6297.
Shors, T. J. & Mathew, P. R. (1998). NMDA receptor antagonism in the lateral/basolateral but not central nucleus of the amygdala prevents the induction of facilitated learning in response to stress.Learn. Mem., 5, 220–230.
Shors, T. J. & Miesegaes, G. (2002). Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood.Proc. Natl. Acad. Sci. U.S.A., 99, 13955–13960.
Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T., & Gould, E. (2001). Neurogenesis in the adult is involved in the formation of trace memories.Nature, 410, 372–376.
Shors, T. J. & Servatius, R. J. (1995). Stress-induced sensitization and facilitated learning require NMDA receptor activation.Neuroreport, 6, 677–680.
Shors, T. J. & Servatius, R. J. (1997). The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning.Neurobiol. Learn. Mem., 68, 92–96.
Shors, T. J., Weiss, C., & Thompson, R. F. (1992). Stress-induced facilitation of classical conditioning.Science, 257, 537–539.
Steenbergen, H. L., Heinsbroek, R. P., van Haaren, F., & van de Poll, N. E. (1989). Sex-dependent effects of inescapable shock administration on behavior and subsequent escape performance in rats.Physiological Behavior, 45, 781–787.
Turnbull, A. V. & Rivier, C. L. (1999). Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action.Physiological Review., 79, 1–71.
Turner, B. B. & Weaver, D. A. (1985). Sexual dimorphism of glucocorticoid binding in rat brain.Brain Research, 343, 16–23.
Viau, V. & Meaney, M. J. (1991). Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat.Endocrinology, 129, 2503–2511.
Watkins, L. R. & Maier, S. F. (2000). The pain of being sick: implications of immune-to-brain communication for understanding pain.Annual Review of Psychology, 51, 29–57.
Wood, G. E., Beylin, A. V., & Shors, T. J. (2001) The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females.Behavioral Neuroscience., 115, 175–187.
Wood G. E. & Shors, T. J. (1998). Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones.Proc. Natl. Acad. Sci. U.S.A., 95, 4066–4071.
Woolley, C. S. & McEwen, B. S. (1992). Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat.Journal of Neuroscience, 12, 2549–2554.
Woolley, C. S. & McEwen, B. S. (1993). Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat.Journal of Comparative Neurology, 336, 293–306.
Yirmiya, R. (1996). Endotoxin produces a depressive-like episode in rats.Brain Research, 711, 163–174.
Yirmiya, R., Avitsur, R., Donchin, O., & Cohen, E. (1995). Interleukin-1 inhibits sexual behavior in female but not in male rats.Brain Behavior Immunology, 9, 220–233.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beck, K.D., Servatius, R.J. Stress and cytokine effects on learning: What does sex have to do with it?. Integrative Physiological & Behavioral Science 38, 179–188 (2003). https://doi.org/10.1007/BF02688852
Issue Date:
DOI: https://doi.org/10.1007/BF02688852