Summary
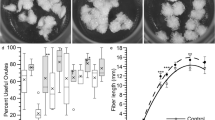
Cotton fibers are often utilized as a model system to investigate cellulose biosynthesis and cell wall elongation. In this study, we grew cotton fibers in vitro, with ovules dissected at day zero post anthesis as the expiant source, in the presence of three herbicides that inhibit cellulose biosynthesis. Cultures were sampled for electron microscopy and immunocytochemistry 1–2 days after beginning the treatments. After dichlobenil treatment, the fibers were much shorter than the controls and assumed a variety of abnormal shapes, from shortened versions of the control fiber to nearly spherical. The inner layers of the fiber wall often contained juxtaposed electron-translucent and -transparent areas; this layer reacted strongly with antibodies to callose. Cellulase-gold labeling in these newly developed fibers grown in the presence of dichlobenil was present at only about 3% of the control labeling. After treatment with either isoxaben or flupoxam, the fibers assumed spherical shapes and frequently (more than 60% of fibers) exhibited a new cell plate within the fiber, indicating that cell division had occurred, a process that rarely occurred in the controls. Unlike the dichlobenil-treated fibers, fibers grown in the presence of isoxaben or flupoxam contained an extensive accumulation of chiefly deesterified pectins, replacing the entire wall with an elaborated version of the pectin sheath found in control cotton fibers. These data indicate that all three herbicides are effective disrupters of cellulose biosynthesis and cause radical changes in cell wall structure and composition. Moreover, these data indicate that the composition of the walls may influence indirectly cell cycle kinetics, keeping these fiber cells in a more meristematic mode.
Similar content being viewed by others
References
Basra AD (2000) Cotton fibers: developmental biology, quality improvement and textile processing. Food Products Press, New York
— Malik CP (1984) Development of the cotton fiber. Int Rev Cytol 89: 65–113
Beasley CH, Ting IP (1973) The effects of plant growth substances on in vitro fiber development from fertilized cotton ovules. Am J Bot 60:130–139
Delmer DP, Cooper G, Alexander D, Cooper J, Hayashi T, Nitsche C, Thelen M (1985) New approaches to the study of cellulose biosynthesis. J Cell Sci Suppl 2: 33–50
De Tullio MC, Paciolla C, Dalla Vacchia F, Rascio N, D’Emerico S, De Gara L, Liso R, Arrigoni O (1999) Changes in onion root development induced by the inhibition of peptidyl-prolyl hydroxylase and influence of the ascorbate system on cell division and elongation. Planta 209: 424–434
Durso NA, Vaughn KC (1997) The herbicidal manipulation of callose levels in cell plates radically affects cell plate structure. Plant Physiol Suppl 114s: 87
Fisher DD, Cyr RJ (1998) Extending the microtubule/microflbril paradigm: cellulose synthesis is required for normal cortical microtubule alignment in elongation cells. Plant Physiol 116: 1043–1051
Giddings TH, Staehelin LA (1991) Microtubule-mediated control of microfibril deposition: a re-examination of the hypothesis. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, New York, pp 85–99
Haigler CH, Rao NR, Roberts EM, Huang LY, Upchurch D, Trolinder NL (1991) Cultured ovules as models for cotton fiber development under low temperatures. Plant Physiol 95: 88–96
Heim DR, Skomp JR, Tschabold EE, Larrinua IM (1990) Isoxaben inhibits the synthesis of acid-insoluble cell wall materials inArabidopsis thaliana. Plant Physiol 93: 695–700
— Larrinua IM, Murdoch MG, Roberts JL (1998) Triazofenamide is a cellulose biosynthesis inhibitor. Pestic Biochem Physiol 59: 163–168
Knox JP (1997) The use of antibodies to study the architecture and developmental regulation of plant cell walls. Int Rev Cytol 171: 79–120
McCann MC, Roberts KR (1991) Architecture of the primary cell wall. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, New York, pp 109–129
Meekes HTHM (1986) Inhibition and recovery of cell wall formation in root hairs ofCeratopteris thalictroides. J Exp Bot 37: 1201–1210
Moore PJ, Staehelin LA (1988) Immunogold localization of the cell-wall-matrix polysaccharides rhamnogalacturonan I and xyloglucan during cell expansion and cytokinesis inTrifolium pratense L.: implications for secretory pathways. Planta 174: 433–445
Norman MA, Smeda RJ, Vaughn KC, Fuerst EP (1994) Differential movement of paraquat in resistant and sensitive biotypes ofConyza. Pestic Biochem Physiol 50:31–42
Ryser U (1985) Cell wall biosynthesis in differentiating cotton fiber. Eur J Cell Biol 39:236–256
Sabba RP, Vaughn KC (1999) Cellulose biosynthesis inhibitor herbicides. Weed Sci 47: 757–763
— Durso NA, Vaughn KC (1999) Structural and immunocytochemical characterization of the walls of dichlobenil-habituated BY-2 cells. Int J Plant Sci 160: 275–290
Samuels AL, Giddings TH, Staehelin LA (1995) Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol 130:1345–1357
Seagull RW (1993) Cytoskeletal involvement in cotton fiber growth and development. Micron 24: 643–660
Van’t Hof J, Saha S (1997) Cotton fibers can undergo division. Am J Bot 84:1231–1235
Vaughn KC, Turley RB (1999) The primary walls of cotton fibers contain an ensheathing pectin layer. Protoplasma 209:226–237
— Hoffman JC, Hahn MG, Staehelin LA (1996) The herbicide dichlobenil disrupts cell plate formation: immunogold characterization. Protoplasma 194:117–132
Willats WGT, Limberg G, Bucholt HC, Van Alebeek GJ, Benen J, Christensen TMIE, Vissrer J, Voragen A, Mikkelsen JD, Knox JP (2000) Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr Res 327: 309–320
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vaughn, K.C., Turley, R.B. Ultrastructural effects of cellulose biosynthesis inhibitor herbicide on developing cotton fibers. Protoplasma 216, 80–93 (2001). https://doi.org/10.1007/BF02680135
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02680135