Abstract
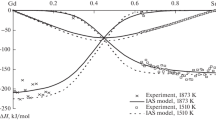
Partial heats of mixing for gadolinium and silicon in the binary system Gd–Si are measured by means of a high-temperature isoperibolic calorimeter in two concentration ranges: 0<xGd<0.2 and 0.8<xGd<1 at 1760 K. The integral enthalpies of mixing are calculated from partial enthalpies by the Darken method. Marked exothermic values of\(\Delta \bar H_{Gd(Si)} \) and ΔH were revealed for these binary alloys. Values of ΔH calculated on the basis of model ideas of the “surrounding atom” theory are in good agreement with experimental values.
Similar content being viewed by others
References
E. M. Savitskii and V. F. Terekhova,REM Physical Metallurgy [in Russian], Nauka, Moscow (1975).
S. Lundin, “Study of the silicides of ytrrium and some rare-earth metals of the yttrium sub-group,” in:New Studies of REM. Series, Metals in New Technology [in Russian], Mir, Moscow (1964).
G. V. Samsonov, P. A. Dvorina, and B. M. Rud’,Silicides [in Russian], Nauka, Moscow (1979).
T. Ya. Kosolapova (ed.),Properties, Preparation, and Use of Refractory Compounds: Handbook [in Russian], Metallurgiya, Moscow (1986).
G. I. Batalin, V. S. Sudavtsova, and N. V. Stroganova, “Enthalpy of formation of liquid binary alloys of the systems Si−La (Gd, Dy, Ho, Er),”Ukr. Khim. Zhurn.,51, No. 7, 775–777 (1985).
S. V. Meschel and O. J. Klappa “Standard enthalpies of formation of some neodymium and gadolinium carbides, silicides and germanides by high-temperature direct synthesis calorimetry,”J. Alloys and Comp.,217, No. 2, 235–239 (1995).
S. V. Meschel and O. J. Klappa, “Standard enthalpies of formation of some lutetium alloys by high-temperature direct synthesis calorimetry,”J. Alloys and Comp.,224, No. 2, 343–350 (1995).
N. P. Gorbachuk, A. S. Bolgar, and A. V. Blinder, “Thermodynamic properties of silicides,”Preprint, Institute for Problems of Materials Science, Ukraine National Academy of Sciences; 95-10, Kiev (1995).
N. J. Usenko, M. I. Ivanov, V. V. Berezutski, and R. J. Polotska, “Calorimetric determination of standard molar enthalpies of formation of gadolinium silicides and germanides,”J. Alloys and Comp.,266, No. 2, 186–190 (1998).
G. M. Lukashenko and R. I. Polotskaya, “Thermodynamic properties of gadolinium disilicide,”Poroshk. Metall., No. 6, 70–72 (1986).
G. N. Lukashenko and R. I. Polotskaya, “Thermodynamic properties of gadolinium silicides GdSi and GdSi1.5,”Poroshk. Metall., No. 9, 75–78 (1992).
G. M. Lukashenko, R. I. Polotskaya, and V. R. Sidorko, “Thermodynamic properties of scandium, lanthanum, neodymium, and gadolinium silicides and germanides,”J. Alloys and Comp.,179, No. 1–2, 295–305 (1992).
V. R. Sidorko and R. I. Polotskaya, “Thermodynamic properties of gadolinium silicides Gd5Si4 and Gd5Si3,”Poroshk. Metall., Nos. 9–10, 93–95 (1993).
S. P. Gordienko and A. G. Druzheruchenko, “Thermodynamic characteristics of high-temperature evaporation in a vacuum of gadolinium silicides,”Poroshk. Metall., Nos. 9–10, 35–38 (1996).
Yu. I. Buyanov, T. Ya. Velikanova, S. P. Luzan, et al., “Features of the reaction of rare-earth metals with silicon,”Preprint, Institute for Problems of Materials Science, Ukraine National Academy of Sciences; 97-5, Kiev (1997).
V. P. Glushko (ed.),Thermal Constants of Substances: Handbook in 10 issues, No. 4, part 1 (1970), No. 8, part 1 (1978), VINITI, Moscow.
I. V. Nikolaenko, M. A. Turchanin, G. I. Batalin, and E. A. Beloborodova, “High-temperature calorimeter for studying the enthalpy of formation of molten metals,”Ukr. Khim. Zhurn.,53, No. 8, 795–799 (1987).
V. N. Eremenko, K. A. Meleshevich, Yu. I. Buyanov, and P. S. Martsenyuk, “Phase equilibrium diagram of the gadolinium-silicon system,”Ukr. Khim. Zhurn.,57, No. 10, 1047–154 (1991).
I. V. Nikolaenko, G. I. Batalin, and E. A. Beloborodova, “Calorimetric study of the formation of liquid alloys of germanium with rare-earth elements,”Dokl. Akad. Nauk UkSSR, Ser. B, No. 12, 51–55 (1980).
V. A. Kireev,Practical Calculation Methods in the Thermodynamics of Chemical Reactions [in Russian], Khimiya, Moscow (1975).
E. A. Belobrodova, “Reaction of the components of binary liquid alloys of germanium withp-, d-, andf-metals of the periodic system,”Diss. Doct. Chem. Sci., Kiev (1987).
A. K. Niessen, F. R. De Beer, R. Boom, et al., “Model predictions for the enthalpy of formation of transition metal alloys. II,”CALPHAD,7, No. 1, 51–70 (1983).
Additional information
Taras Shevchenko Kiev National University. Translated from Poroshkovaya Metallurgiya. Nos. 11–12(410), pp. 81–85, November–December, 1999.
Rights and permissions
About this article
Cite this article
Beloborodova, E.A., Golovataya, N.V., Zinevich, T.N. et al. Thermodynamic properties of liquid alloys of gadolinium with silicon. Powder Metall Met Ceram 38, 603–607 (1999). https://doi.org/10.1007/BF02676194
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02676194