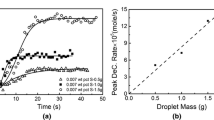
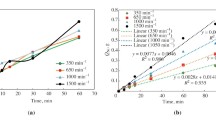
Abstract
Equations have been developed for calculating the fraction of free surface, 1-θT, when two or more surface-active elements (S, O,Se,etc.) are present in liquid iron or its alloys. It is shown that equations of the form k =A Vl-θT -B well describe the essentially linear plots which represent the variation of rate constant k with concentration of surface-active elements in more than 30 researches on absorption and desorption of nitrogen in stirred liquid iron and its alloys. To normalize the effect of variation in metallodynamic properties from researcher to researcher, a second relation has been developedwhich describes a dimensionless rate constant for nitrogen k FN (with range0 to 1) given by k FN = CVl-θT-D, where C and D are small constants. The relation k FN = 1.19 Vl-θT-0.19 is a fair representation of the absorption and desorption behaviors in the 1550 ° to 1600 ° range for all the many iron and iron alloy cases examined. Although these two relations are largely empirical they and the simple linear graphs involved provide potentially valuable new methods of rate prediction. There is some evidence that the behavior of hydrogen with liquid Fe, Cu, and Ni is analogous.
Similar content being viewed by others
References
G.R. Belton:Metall. Trans. B, 1976, vol. 7B, pp. 35–42.
M. T. Utine and N. A. D. Parlee:Trans. TMS-AIME, 1968, vol. 242, pp. 1458–60.
S. Ban-Ya, T. Shinohara, H. Tozaki, and T. Fuwa:Tetsu-to-Hagane, 1974, vol. 160, p. 21.
P. J. Depuydt and N. A.D. Parlee:Metall. Trans., 1972, vol. 3, pp. 525–32.
J. Y. Lee and N. A. D. Parlee:High Temp. Sci., 1972, vol. 4, no. 2, pp. 147–59.
V. G. Levich: “Physicochemical Hydrodynamics”, Prentice Hall, Englewood Cliffs, NJ, 1962, pp. 689–99.
R.D. Pehlke and J.F. Elliott:Trans. TMS-AIME, 1963, vol. 277, pp. 844–55.
W. M. Boorstein and R.D. Pehlke:Trans. TMS-AIME, 1969, vol. 245, p. 1843.
R.D. Pehlke and J.F. Elliott:Trans. TMS-AIME, 1963, vol. 277, p. 844.
R.G. Mowers and R.D. Pehlke:Metall. Trans., 1970, vol. 1, p. 51.
M. Inouye and T. Choh:Trans. ISIJ, 1968, vol. 8, p. 134.
S. Ban-ya, T. Sinohara, H. Tozaki, and T. Fuwa:Tetsu-to-Hagane, 1974, vol. 60, p. 21.
T. Choh, T. Moritani, and M. Inouye:Trans. ISIJ, 1979, vol. 19, p. 221.
K. Narita, S. Oyama, T. Makino, and M. Okamura:Tetsu-to-Hagane, 1971, vol. 57, p. 2207.
P. Kosakevitch and G. Urbain:Mem. Scientif. de la Revue de Metl., 1963, vol. 60, pp. 143–56.
W. Small, R. Radzilowski, and R.D. Pehlke:Metall. Trans., 1973, vol. 4, p. 2045.
V.I. Fedorchenko and V. V. Avarin: “Russian Metallurgy”, 1974, vol. 3, p. 26.
M. Inouye and T. Choh:Trans. ISIJ, 1972, vol. 12, p. 189.
M. Inouye, T. Choh, and Yamada:Tetsu-to-Hagane, 1976, vol. 62, p. 334.
K. Suzuki, K. Mori, and Y. Ito:Tetsu-to-Hagane, 1969, vol. 55, p. 877.
Fuwa,et al:Tetsu-to-Hagane, 1967, vol. 53, p. 5328.
M. Inouye, M. Takada, and T. Choh:Trans. ISIJ, 1977, vol. 17, p. 653.
C.H. Hua: “Prediction of Solubilities, Diffusivities, and Effects of Surface Active Elements on Kinetics of Gases in Liquid Iron Alloys”, Ph.D. Dissertation, Stanford University, Stanford, CA, 1980.
H.J. Grabke, W. Paulitschke, and H. Veifhaus:Surface Science, 1977, vol. 63, p. 377.
G.M. Grigorenko: Paton Electric Welding Institute (USSR), private communication under the US-USSR cooperative research program on “Electrometallurgy and Materials”, 1976.
R.J. Fruehan and L. J. Martonik:Metall. Trans. B, 1980, vol. 11B, pp. 615–21.
P.V. Danckwerts:Ind. Engng. Chem., 1951, vol. 43, p. 1460.
L.J. Austin and H. Sawiwsotwki: Inst. Chem. Engrs., London, Symp. Ser. No. 26, 1967, p. 3.
R. J. Fruehan and L. J. Martonik:Metall. Trans. B, 1981, vol. 12B, pp. 379–84.
F.D. Richardson:Physical Chemistry of Melts in Metallurgy, Aca-demic Press, London and New York, NY, 1974, vol. 2, pp. 408–16, 426–39.
J. T. Davies and W. Khan:Chem. Engng. Sci., 1962, vol. 20, p. 713.
M. Byrne and G.R. Belton: Ph.D. Thesis Research, University of Pennsylvania (private communication from reviewer), 1981.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hua, C.H., Parlee, N.A.D. Prediction of the effects of surface-active elements on gas-liquid metal kinetics. Metall Trans B 13, 357–367 (1982). https://doi.org/10.1007/BF02667751
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02667751