Abstract
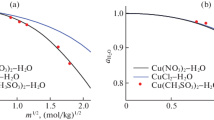
The known thermodynamic data for the aqueous zinc and sulfur dioxide systems have been gathered, evaluated, and presented in the form of potential -pH diagrams. Empirical relationships describing the entropy of ions at 298 K combined with the extrapolation method of Criss and Cobble[6] have been used in the absence of high-temperature free-energy data. The free energy of formation of zinc sulfite (ZnSO3 · 2.5H2O) has been experimentally determined to be −1256 ± 4 kJ mol−1 and has been incorporated into the diagrams along with the various metastable polythionate species of sulfur.
Similar content being viewed by others
Abbreviations
- (i):
-
activity of speciesi
- C o p :
-
standard molal heat capacity
- C o p :
-
standard mean molal heat capacity
- Eh :
-
potential of electrode referred to SHE
- F:
-
the Faraday
- ΔG o p :
-
standard free energy of formation of species
- ΔG o :
-
standard free energy change of reaction
- K :
-
equilibrium constant or solubility product
- n :
-
number of electrons involved in reaction
- R:
-
gas constant
- S o 298 :
-
molal entropy of species in standard state at 298.15 K
- SHE:
-
standard hydrogen electrode
- T :
-
absolute temperature
References
M. Pourbaix:Atlas of Electrochemical Equilibria in Aqueous Solutions, Pergamon, Oxford, United Kingdom, 1966.
J.D. Esdaile and G.W. Walters: CSIRO Australia, Report R17, 1969.
R.B. Sudderth, J.B. Clitheroe, and G.A. Kordosky: General Mills Chemicals, Inc., AIME Meeting, Denver, CO, Feb. 1978.
Gmelins Handbuch der Anorganischen Chemie, Zink-erg, Verlag Chemie - GMBH, 1956, vol. 32, p. 935.
F.T. Heuston and C.R. Tichborne:Brt. Med. J., 1890, p. 1063.
C.M. Criss and J.W. Cobble:J. Am. Chem. Soc, 1964, vol. 86, p. 5385.
P.P. Wagman, W.H. Evans, V.B. Parker, R.H. Schumm, I. Halow, S.M. Bailey, K.L. Cumey, and R.L. Nuttal:J. Phys. Chem. Ref. Data, The ACS and The NBS, Washington, DC, 1982, vol. 11 (2).
G.B. Naumov, B.N. Ryzhenko, and I.L. Khodakovsky:Handbook of Thermochemical Data, United States Dept. of Commerce, Washington, DC, 1974.
D.M. Larsen: Ph.D. Thesis, The University of Sydney, Sydney, Australia, 1985.
W.M. Latimer:J. Am. Chem. Soc, 1951, vol. 73, p. 1480.
J.W. Cobble:J. Chem. Phys., 1953, vol. 21, p. 1446.
A.M. Couture and K.J. Laidler:Can. J. Chem., 1957, vol. 35, p. 202.
P.B. Linkson, B.D. Phillips, and C.D. Rowles:Minerals Sci. Eng., 1979, vol. 11, p. 65.
O.J. Kwok and R.G. Robins:Int. Symp. on Hydrometallurgy, D.J.I. Evans and R.S. Shoemaker, eds., AIME, New York, NY, 1973, pp. 1033–80.
L.C. Schroeter:Sulphur Dioxide, Pergamon, London, 1966.
H. Debus:Trans. J. Chem. Soc, 1888, vol. 53, pp. 278–357.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Larsen, D.M., Linkson, P.B. Thermodynamics of the zinc-sulfur dioxide-water system. Metall Trans B 24, 409–417 (1993). https://doi.org/10.1007/BF02666423
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02666423