Conclusions
-
1.
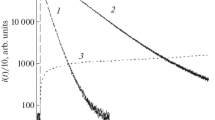
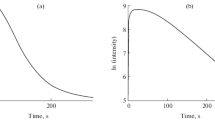
Spectrophotometric studies have been carried out on the oxidation of luminol by sodium hypochlorite, working with solutions of various concentrations and pH values. Formation of the final product is the result of a series of successive reactions. The limiting step in the process is the two-electron oxidation of luminol (monoanion) by hypochlorous acid with the formation diazoquinone.
-
2.
The bimolecular rate constant for the limiting step has been evaluated.
-
3.
It has been shown that an unstable intermediate, blue-violet in color (pH<9 nm), is formed in the reaction at pH<9. It is assumed that this is a complex of diazoquinone with HOCl which is rapidly converted to stable products.
-
4.
A parallel path for diazoquinone consumption would lead through hydrolysis and/or a series of sequential reactions, one of which would involve O2. Chemiluminescence arises in the course of this, sequence of changes.
Similar content being viewed by others
Literature cited
H. O. Albrecht, Z. Phys. Chem.,136, 321, (1928).
A. K. Babko, L. I. Dubovenko, and N. M. Lukovskaya, Chemiluminesence Analysis [in Russian], Tekhnika, Kiev (1966).
G. Sharlo, Methods of Analytical Chemistry, Quantitative Analysis of Inorganic Compounds [Russian translation], Khimiya, Moscow (1969), p. 1129.
S. M. Osinkina-Tanevska, M. K. Bynyaeva, K. P. Mishchenko, and Sh. E. Flis, Zh. Prikl. Khim.,36, 1212 (1963).
A. S. Shalomeev, T. I. Smol'yaninova, E. M. Gonikberg, E. M. Brazhnikov, E. K. Russiyan, and V. M. Andreev, Izv. Akad. Nauk SSSR, Ser. Khim., 1035 (1970).
M. Kh. Karapet'yants and M. L. Karapet'yants, Basic Thermodynamic Constants for Organic and Inorganic Compounds [in Russian], Khimiya (1968).
A. D. Nadezhdin, Yu. N. Kozlov, and A. P. Purmal', Zh. Fiz. Khim.,49, 2263 (1975).
R. A. Clement, J. Org. Chem.,26, 1724 (1960).
T. J. Kealy, J. Am. Chem. Soc.,84, 966 (1962).
L. Farkas, M. Lewin, and R. Bloch, J. Am. Chem. Soc.,71, 1988 (1949).
G. E. Lewis, Tetrahedron,10, 129 (1960).
Additional information
Institute of Chemical Physics, Academy of Sciences of the USSR, Moscow. Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 10, pp. 2187–2193, October, 1976.
Rights and permissions
About this article
Cite this article
Vorob'eva, T.P., Kozlov, Y.N., Koltypin, Y.V. et al. Processes involved in luminol oxidation accompanied by chemiluminescence. Russ Chem Bull 25, 2043–2048 (1976). https://doi.org/10.1007/BF02659512
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02659512