Abstract
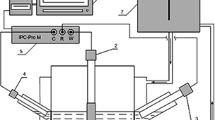
An apparatus was built in our laboratory in order to electrolyze a Gokhale solution (CuC10.7N,NaC14N, HC10.5N)for extended periods of time under current densities ranging from 50 to 600 A/m2 at temperatures between 20 and 50°C The influence of the diaphragm, temperature, current density and duration of the electrolysis in pure solutions was investigated, and the results were interpreted in terms of current efficiency, overall voltage drop and specific energy consumption. The structure of the deposits was investigated by profilometry, optical micrography, scanning electron microscopy and X-ray diffraction. The structure of the deposits is not significantly different from that obtained in pure sulfuric acid solutions, but it shows an increased tendency to growth of rather large elongated crystals protruding in the electrolyte. Both types are characteristic of deposits obtained at rather low inhibition, according to Fischer’s classification.
Similar content being viewed by others
References
E. A. Ashcroft:Trans. Electrochem. Soc, 1933, vol. 63, p. 23.
S. D. Gokhale:J. Sci. Ind. Res., 1951, vol. 10B,p.316.
G. C. Mitter, B. K. Rose, S. G. Dighe, Y. W. Gokhale, and B. P. Choudhury:J. Sci. Ind. Res., 1961, vol. 20D, p. 114.
V. Aravamuthan and R. Srinivasan:J Sci. Ind. Res., 1962, vol. 21D, p. 381.
P. R. Kruesi: U.S. Patent 3,673,061, 1972.
G. E. Atwood and C. H. Curtis: U.S. Patent 3, 785, pp944, 1974.
R. E. Mussler, R. S. Olsen, and T. T. Campbell: U.S. Bureau of Mines Report of Investigation 8076, 1975.
K. J. Cathro:Extractive Metallurgy of Copper-A Symposium Held During the 105th AIME Annual Meeting, p. 776, Las Vegas, February, 1976.
V. Engelhardt and M. Hosenfeld:Metall Erz, 1922, vol. 19, p. 508.
G. Hansel:Wiss. Veroeff. Siemens-Konzern, 1925, vol. 4, p. 111.
Noata Kameyama and Tokichi Noda:J Inst. Metals, 1928. vol. 40, p. 642.
N. P. Diep and A. G. Loshkarev:J. Appl. Chem., (USSR), 1939, vol. 12, pp. 585 and 592.
R. Winand:Tram. Inst. MiningMet., 1975, vol. 84, p. C67.
P. Andrianne: Graduate Thesis, Free University of Brussels, 1974.
J. Besson and J. Guiton:Manipulations d’Electrochimie, Masson et Cie, Paris, 1972.
P. Duby: Private communication, (see also Choi’s Ph.D. Thesis, Columbia University, 1976).
Author information
Authors and Affiliations
Additional information
Formerly Researcher, Université Libre de Bruxelles
Rights and permissions
About this article
Cite this article
Andrianne, P.A., Dubois, J.P. & Winand, R.F.P. Electrocrystallization of copper in chloride aqueous solutions. Metall Trans B 8, 315–321 (1977). https://doi.org/10.1007/BF02657662
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02657662