Abstract
The free energy of mixing in the Mn-P melts in the composition range ofX p = 0.0 to 0.333 was estimated by coupling the phase boundary information with reliable ΔG° formation for the Mn2P phase. This information was used to obtain the dilute solution properties of P in Mn. P(l,pure) = P(l,Henrian, Mn) ΔG °(Joules) = -203,611.39 + 41.003T The free energy is shown to be more negative than in the Fe system, reflecting a stronger interaction between Mn and P atoms than between Fe and P atoms. Presenting the activity coefficient of P with the expression used by Lupis and Elliott, the first and second interaction coefficients are obtained as follows: ε PP (Mn) = 10.538 + 9728.14/T ρ PP (Mn) = 28.148 + 9101.83/T The Gibbs free energy of formation for Mn3P was estimated in the temperature range of {dy1233} to {dy1378} K to be 3Mn l + P(l = Mn3P(s ΔG °(Joules) = -241,461.65 + 65.031T
Similar content being viewed by others
References
S.K. Sigworth and J. F. Elliott:Metal Science, 1974, vol. 8, pp. 298–310.
J.K Elliott, M. Gleiser, and M. Ramakrishna:Thermochemistry for Steelmaking, Addison-Wesley Pub., Reading, MA, 1963, vol. II, pp. 564–67.
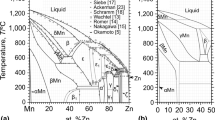
M. Hansen:Constitution of Binary Alloys, 2nd ed., McGraw-Hill, New York, NY, 1958, pp. 941–43.
P. Spencer and O. Kubaschewski:Arch. Eisenhüttenwes., 1978, vol. 49, pp. 225–28.
L. B. Pankratz:Thermodynamic Properties of Elements and Oxides, U.S. Bureau of Mines, Bulletin 672, 1982, p. 229.
D. R. Stall and H. Prophet:JANAF Thermochemical Tables, 2nd ed., U.S. National Bureau of Standards, NSRDS-NBS 37, 1971.
I.B. Baratashvili, D. Sh. Tsagareishvili, and I.N. Dashniami:Soobshch. Akad. Nauk. Gruz. SSR., 1980, vol. 99, pp. 121–23.
I. Barin, O. Knacke, and O. Kubaschewski:Thermochemical Properties of Inorganic Substances, Berlin/Heidelburg/New York and Düsseldorf, 1977, p. 403.
I. A. Makharadze, I. B. Baratashvili, D. Sh. Tsagareishvili, and G. G. Gvelesiani:Izv. Akad. Nauk SSSR, Neorg. Mater., 1975, vol. 11, pp. 599–601.
F. Grandjean, D.W. Osborne, W.G. Lyon, and H.E. Flotow:J. Chem. Thermodyn., 1977, vol. 9, pp. 549–59.
I.B. Baratashvili, A.A. Nadiradze, I.A. Makharadze, and L.A. Shvartsman:Dok. Akad. Nauk SSSR, 1975, vol. 224, pp. 844–46.
C. E. Myers, E. D. Jung, and E. L. Patterson:Inorg. Chem., 1980, vol. 19, pp. 532–34.
V. K. Tagirov, E. K. Kazenas, V. Ya. Dashevskii, V. I. Kashin, and N. I. Rakitina:Fiz.-Khim. Osn. Metall. Margantsa, L. M. Tsylev, ed., Nauka, Moscow, USSR, 1975, 1st, pp. 5–12.
D. M. Chizhikov, V. I. Kashin, E. K. Kazenas, V. K. Tagirov, V. Ya. Dashevskiy, and N. I. Rakitina:Russ. Metall. (Metally), 1975, No. 5, pp. 52-56 (English), p. 64 (Russian).
G.I. Batalin, V. A. Stukalo, and N. Ya. Neshchimenko:Ukr. Khim. Zh., 1977, vol. 43, pp. 602–04.
V.Ya. Dashevskii, V.I. Kashin, N.I. Rakitina, and M.S. Ageev:Zakonomern. Vzaimodeistviya Zhidk. Met. Gazami Shlakami, I. S. Kulikov, ed., Nauka, Moscow, USSR, 1976, pp. 109–13.
C.H.P. Lupis and J.F. Elliott:Acta Metall., 1966, vol. 14, pp. 529–38.
S.A. Shchukarev, N.M. Morozova, and T.A. Stolyarova:Zh. Obshch. Khim., 1961, vol. 31, No. 7, p. 1773.
W. Biltz, F. Wiechman, and K. Meizel:Z. Anorg. Allgem. Chem., 1937, vol. 234, p. 117.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, Y.E. Thermodynamics of the Mn-P system. Metall Trans B 17, 777–783 (1986). https://doi.org/10.1007/BF02657140
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02657140