Abstract
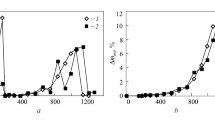
Solid Cu-S-O alloy (about 150 g) containing 0.05 pct S and 0.04 pct O (by weight) was heated to 1473 or 1573 K under argon at 6.5 to 101 kPa. Desulfurization of 64 to 80 pct and deoxidation of 75 to 82 pct were observed. The extent did not depend on argon pressure, and this desulfurization and deoxidation probably occurred during melting. In the course of solidification and remelting of molten Cu-S-O alloys containing sulfur and oxygen at approximately the same concentration,i.e., 0.027 pct S and 0.026 pct O, and 0.0456 pct S and 0.0416 pct O under argon at 1.33 to 102 kPa, the percentage of sulfur removed was 49 to 75, the percentage of oxygen removed was 63 to 81, and the extent did not depend on argon pressure. The alloys containing more oxygen than sulfur before cooling,i.e., 0.0216 pct S and 0.081 pct O and 0.0185 pct S and 0.15 pct O, 73 to 90 pct of the sulfur was eliminated. Most desulfurization and deoxidation probably occurred in the course of solidification, because vigorous boiling was observed at this stage. The rates of removal of oxygen and sulfur from molten Cu-S-O alloys maintained at 1373, 1473, and 1573 K under vacuum were expressed by a first-order rate equation, and the dependence of their rate constants on the reciprocal of temperature was determined. In alloys in which pct S ≃pct O before evacuation and pct S ranges from 0.0202 to 0.042 and pct O ranges from 0.023 to 0.048, 79 to 96 pct desulfurization and 66 to 87 pct deoxidation were observed. For the alloys with pct O > pct S initially,i.e., 0.081 pct O and 0.0216 pct S, and 0.15 pct O and 0.0176 pct S, 85 to 94 pct desulfurization was observed and was close to the observed levels of desulfurization in solidification and remelting of the alloys with pct O > pct S under argon.
Similar content being viewed by others
References
M. Kameda and A. Yazawa:Tohoku Daigaku Senko Seiren Kenkyusho Iho, 1963, vol. 19, pp. 57–68.
H. Kametani and C. Yamauchi:Trans. Jpn. Inst. Met., 1972, vol. 13, pp. 13–20.
H. Kametani and C. Yamauchi:Nippon Kogyo Kaishi, 1972, vol. 88, pp. 347–52.
CF. Floe and J. Chipman:Trans. AIME, 1942, vol. 147, pp. 28–38.
O. Knacke and H. Probst: inMetallurgical Chemistry, O. Kubaschewski, ed., Her Majesty’s Stationary Office, London, 1972, pp. 527–31.
T. Ejima, N. Inagaki, and M. Kameda:Trans. Jpn. Inst. Met., 1966, vol. 7, pp. 133–42.
Shinya Otsuka and Zensaku Kozuka:Metall. Trans. B, 1976, vol. 7B, pp. 147–49.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ohno, R. Desulfurization and deoxidation of Cu-S-O alloy in induction melting and solidification under argon and their rates of elimination in vacuum induction melting. Metall Trans B 22, 405–416 (1991). https://doi.org/10.1007/BF02654279
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02654279