Abstract
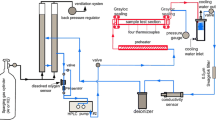
The reaction of molten Al-2.5 pct Li alloy or pure aluminum with water was studied by the exploding wire technique incorporating a crowbar switch current interruptor and an inductive energy store. The extent of chemical reaction was measured by the amount of hydrogen gas product vs the electrical energy input to the alloy or Al wire placed in water. The minimum electrical energy required to trigger the chemical reaction of the molten alloy or Al with water was about 2 kJ per gram of the metal. A further increase in the energy input to the wire revealed a large difference in the degree of chemical conversion between the two metals; the alloy wire needed only 4 kJ/g of the energy to achieve more than 90 pct of chemical conversion, whereas pure aluminum wire to achieve the same degree of the conversion needed 10 kJ/g to vaporize most of the wire mass. The higher reactivity of the alloy is interpreted as a result of the role of lithium in the alloy in altering the structure and strength of the surface oxide layer and increasing the oxide formation rate whereby the mixing of the liquids is facilitated.
Similar content being viewed by others
References
R. C. Reid:Am. Sci., 1976, vol. 64, pp. 146–56.
L. C. Witte and J. E. Cox:J. Metals, 1978, vol. 30, pp. 29–35.
G. B. Usynin and N. I. Khramov:Fizica Goreniya i Vzryva, 1985, vol. 21, pp. 117–20.
L. S. Nelson:SAND-80-0803C, Sandia Nat’l Lab., Albuquerque, NM, 1980.
H. S. Levine:J. Phys. Chem., 1972, vol. 76, pp. 2609–14.
D. J. Field and E. P. Butler:Al-Li Alloys II, E. A. Starke and T. H. Sanders, eds., AIME, Warrendale, PA, 1983, pp. 667–73.
W. Kahl and E. Fromm:Metall. Trans. B, 1985, vol. 16A, pp. 47–51.
L. Leibowitz and L.W. Mishler:J. Nucl. Mat., 1967, vol. 23, pp. 173–82.
L. Baker:Nucl. Sci. Eng., 1966, vol. 25, pp. 116–30.
M. Friedman and M. Ury:Rev. Sci. Instrum., 1977, vol. 48, pp. 279–81.
W. M. Lee and R. D. Ford:NSWC TR 86-78, Naval Surface Weapons Center, MD, 1986.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, W.M., Ford, R.D. Reactivity of Al-2.5 Pct Li alloy with water as studied by the exploding wire technique. Metall Trans B 19, 255–259 (1988). https://doi.org/10.1007/BF02654210
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02654210