Abstract
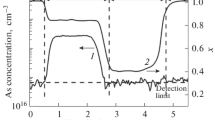
A dilute mixture of CCl4 in H2 has recently been shown to be a suitable carbon doping source for obtainingp-type GaAs grown by metalorganic chemical vapor deposition (MOCVD) with carbon acceptor concentrations in excess of 1 × 1019cm−3. To understand the effect of growth parameters on carbon incorporation in CCl4 doped Al x Ga1−x As, carbon acceptor concentration was studied as a function of Al composition, growth temperature, growth rate, and CCl4 flow rate using electrochemical capacitance-voltage profiling. The carbon incorporation as a function of Al composition, growth temperature and CCl4 flow rate was also measured by secondary ion mass spectroscopy (SIMS). All layers were grown by low pressure MOCVD using TMGa and TMAl as column III precursors, and 100% AsH3 as the column V source. Increased Al composition reduced the dependence of carbon concentration on the growth temperature. Reduced growth rate, which resulted in substantially decreased carbon acceptor concentrations in GaAs, had an insignificant effect on the carrier concentration of Al0.4Ga0.6As. A linear relationship between hole concentration and CC14 flow rate in AlxGa1−x As for 0.0 ≤x ≤ 0.8 was observed. These results are interpreted to indicate that adsorption and desorption of CCl y (y ≤ 3) on the Al x Ga1-x As surface during crystal growth plays an important role in the carbon incorporation mechanism.
Similar content being viewed by others
References
B. T. Cunningham, M. A. Haase, M. J. McCollum, J. E. Baker and G. E. Stillman, Appl. Phys. Lett.54, 1905 (1989).
T F. Kuech, M. A. Tischler, P.-J. Wang, G. Scilla, R. Potemski and F. Cardone, Appl. Phys. Lett.53, 1317 (1988).
T. F. Kuech and E. Veuhoff, J. Cryst. Growth68, 148 (1984).
R. M. Lum, J. K. Klingert, D. W. Kisker, D. M. Tennant,M. D. Morris, D. L. Malm, J. Kobalchick and L. A. Heimbrook, J. Electron. Mater.17, 101 (1988).
L. J. Guido, G. S. Jackson, D. C. Hall, W. E. Piano and N. Holonyak, Jr., Appl. Phys. Lett.52, 522 (1988).
K Tamamura, J. Ogawa, K. Akimoto, Y. Mori and C. Kojima, Appl. Phys. Lett.50, 1149 (1987).
B. T. Cunningham, L. J. Guido, J. E. Baker, J. S. Major, Jr.,N. Holonyak, Jr. and G. E. Stillman, Appl. Phys. Lett.55,687 (1989).
L. J. Guido, B. T. Cunningham, D. W. Nam, K. C. Hsieh, W. E. Piano, J. S. Major, Jr., E. J. Vesely, A. R. Sugg, N. Holonyak, Jr. and G. E. Stillman, unpublished.
P. M. Enquist, J. Cryst. Growth93, 637 (1988).
B. T. Cunningham, and G. E. Stillman, unpublished.
B. T. Cunningham, G. S. Jackson, and G. E. Stillman, unpublished.
T. F. Kuech, D. J. Wolford, E. Veuhoff, V. Deline, P. M. Mooney, R. Potemski and J. Bradley, J. Appl. Phys.62, 632 (1987).
K. P. Schug et al., Ber. Bunsenges Phys. Chem.83,167 (1979).
Handbook of Chemistry and Physics, 1987–1988 Edition, p.F181; and D. R. Stull and H. Prohet, Nat. Std. Ref. Data Series, NBS, No. 37 (1971).
S. J. W. Price, in Comprehensive Chemical Kinetics, Vol. 4,eds. C. H. Bamford, and C. F. H. Tipper (Elsevier Publishing Company, New York, 1972), pp. 197–259.
H. A. Skinner, in Advances in Organometallic Chemistry, Vol. 2, eds. F. G. A. Stone and R. West (Academic Press, New York, 1964), pp. 49–114. (Ref. 3).
T. L. Cottrell, The Strength of Chemical Bonds (Butterworth, London, 1954).
C. T. Mortimer and P. W. Sellers, J. Chem. Soc. 1978 (March 1963).
L. H. Long, Pure Appl. Chem.2, 61 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cunningham, B.T., Baker, J.E. & Stillman, G.E. Carbon tetrachloride doped Al x Ga1−x As grown by metalorganic chemical vapor deposition. J. Electron. Mater. 19, 331–335 (1990). https://doi.org/10.1007/BF02651293
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02651293