Abstract
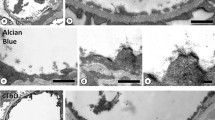
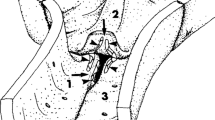
We extended the model describing the low molecular weight electron dense tracer wake in the interendothelial cleft and surrounding tissue to describe the time-dependent transport of intermediate size solutes of 1.0–3.5 nm radius by convection and diffusion in an interendothelial cleft containing a fiber matrix. This model provides a quantitative basis on which to reinterpret electron microscopic studies of the distribution of tracers such as horseradish peroxidase (HRP; molecular weight=40,000; Stokes radius =3.0 nm) along the interendothelial cell cleft from the lumen to the tissue for example, we show that, in contrast to our results with low molecular weight tracers, the wake of large molecular weight tracers on the abluminal side of the junctional strand is not likely to be detected, because the concentration of the tracer is predicted to be very low in most experiments. Thus the lack of a tracer such as HRP on the abluminal side of the junctional strand and in the tissue is not as strong evidence against the presence of a cleft pathway as suggested previously. The model does provide the basis for the design of experiments to locate both the principal molecular sieve and breaks in the junctional strand from the standing gradient on the luminal side of the junctional strand. An important experimental variable is the pressure in the vessel lumen which can be varied between 0 and 30 cm H2O to change the contributions of diffusive and convective transport to transcapillary exchange through the interendothelial cleft. This approach will also allow the testing of models for transcapillary pathways for large molecules by measuring the distribution of fluorescent tracers across the microvessel wall and in the tissue surrounding the microvessel using confocal microscopy.
Similar content being viewed by others
Abbreviations
- a :
-
fiber radius
- B :
-
half height of the cleft
- b :
-
half height of the basic large orifice
- b s :
-
half height of the continuous narrow slit in the junction strand
- C (i) :
-
concentration in cleft regioni, i=1, 3
- C t :
-
concentration in the tissue space
- C L :
-
concentration in the lumen
- C a :
-
tissue concentration at the vessel wall
- D :
-
half distance between the centers of adjacent openings
- D (i) :
-
effective diffusivity in regioni of the cleft,i=1, 3
- D free :
-
free solute diffusivity
- D iw :
-
diffusivity in the restricted region without fiber matrix
- D i,eff :
-
effective diffusivity in the region with fiber matrix
- D t :
-
diffusivity in the tissue
- d :
-
half width of the basic large orifice
- Ei:
-
exponential integral
- H :
-
height of the tissue space
- K :
-
complete elliptic integral of the first kind
- L :
-
total cleft depth
- L B :
-
characteristic length of the intermediate region B
- L i :
-
depth of the cleft regioni, i=1, 2, 3
- L f :
-
depth of the entrance fiber layer
- N c :
-
number of clefts on half surface of the vessel
- P (i) :
-
equivalent permeability in cleft regioni, i=1, 3
- P i,eff :
-
effective permeability in the fiber filled region of the cleft
- P iw :
-
permeability in the cleft region without fiber
- p (i) :
-
pressure in cleft regioni, i=1, 3
- p L :
-
pressure in the lumen
- p A :
-
pressure in the tissue
- Pe(i) :
-
Peclet number in cleft regioni, i=1, 3
- Pe(t) :
-
Peclet number in the tissue
- Q :
-
total flux per unit tissue thickness
- Q c :
-
averaged flux from a single cleft
- q :
-
source strength per unit cleft length
- r :
-
coordinate in the intermediate region
- r m :
-
solute radius
- S f :
-
solid fraction of the fiber matrix
- T :
-
characteristic time
- t :
-
time
- u 0,v 0 :
-
velocity components inx, y directions atz=0 plane
- V 0 :
-
velocity atz=0 plane
- V a :
-
averaged velocity in the junction orifice
- x, y, z :
-
coordinate system
- x t :
-
coordinate in the tissue space
- α:
-
cleft exit coefficient
- β:
-
D (3)/D (1)
- γ:
-
cosh (πd/L)
- λn :
-
eigenvalue=nπ/D, n=1,2,3...
- μ:
-
viscosity
- μ(i) :
-
equivalent viscosity in cleft regioni, i=1, 3
- μeff :
-
effective viscosity in the fiber region
- σ(i) :
-
equivalent reflection coefficient in regioni, i=1, 3
- σ i,eff :
-
effective reflection coefficient in the fiber filled cleft region
- σ iw :
-
effective reflection coefficient in the cleft region without fiber
- Φ(i) :
-
velocity potential in cleft regioni, i=1, 3
- Φ L :
-
velocity potential atx=L
- Φ L 1 :
-
velocity potential atx=L 1
- χ(i) :
-
equivalent retardation coefficient in cleft regioni, i=1, 3
- χt:
-
effective retardation coefficient in the tissue
- ψ(i) :
-
stream function in cleft regioni, i=1, 3
- ψ D :
-
stream function aty=D
- ω k :
-
kπ/ψ D ,k=1, 2, 3, ...
- Δ:
-
gap distance between the fibers
- ≈:
-
variable with dimension
References
Abramowitz, M. and I. A. Stegun.Handbook of Mathematical Functions with Formulas, Graphs and Mathematical Tables. New York: Dover Publications Inc., 1970, pp. 1019–1030.
Adamson, R. H. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx.J. Physiol. 428:1–13, 1990.
Adamson, R. H. An extension of the fiber matrix model of vascular permeability.Microvasc. Res. 43:352–356, 1992.
Adamson, R. H., and G. Clough. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels.J. Physiol. 445:473–486, 1992.
Adamson, R. H., and C. C. Michel. Pathways through the intercellular clefts of frog mesenteric capillaries.J. Physiol. 466:303–327, 1993.
Adamson, R. H., J. F. Lenz, and F. E. Curry. Quantitative laser scanning confocal microscopy on single capillaries: permeability measurement.Microcirculation 1:251–265, 1995.
Boussinesq, M. J. Calculation of cooling power of fluid flows.J. Math. Pure Appl. 1:285–332, 1905.
Bundgaard, M. The three-dimensional organization of tight junctions in a capillary endothelium revealed by serial-section electron microscopy.J. Ultrastruct. Res. 88:1–17, 1984.
Clough, G., and C. C. Michel. Quantitative comparisons of hydraulic permeability and endothelial intercellular cleft dimensions in single frog capillaries.J. Physiol. 405:563–576, 1988.
Crone, C., and D. Levitt. Capillary permeability to small solutes. In:Handbook of Physiology, seet. 2, vol. 4, edited by E. M. Renkin and C. C. Michel. Bethesda, MD: American Physiological Society, 1984, pp. 411–466.
Curry, F. E., J. C. Mason, and C. C. Michel. Osmotic reflexion coefficients of capillary walls to low molecular weight hydrophilic solutes measured in single perfused capillaries of frog mesentery.J. Physiol. 261:319–336, 1976.
Curry, F. E. Permeability coefficients of the capillary wall to low molecular weight hydrophilic solutes measured in single perfused capillaries of frog mesentery.Microvasc. Res. 17:290–308, 1979.
Curry, F. E., and C. C. Michel. A fiber matrix model of capillary permeability.Microvasc. Res. 20:96–99, 1980.
Curry, F. E. Transcapillary exchange. In:Handbook of Physiology, sect. 2, vol. 4, edited by E. M. Renkin and C. C. Michel. Bethesda, MD: American Physiological Society, 1984, pp. 309–374.
Curry, F. E. Determinants of capillary permeability: A review of mechanisms based on single capillary studies in the frog.Circ. Res. 59:367–380, 1986.
Curry, F. E. Regulation of water and solute exchange in microvessel endothelium: Studies in single perfused capillaries.Microcirculation 1:11–26, 1994.
Firth, J. A., K. F. Bauman, and C. P. Sibley. The intercellular junctions of guinea-pig placental capillaries: A possible structure basis for endothelial solute permeability.J. Ultrastruct. Res. 85:45–57, 1983.
Fox, J. R., and H. Wayland. Interstitial diffusion of macromolecules in the rat mesentery.Microvasc. Res. 18:255–276, 1979.
Frøkjær-Jensen, J. The endothelial vesicle system in cryofixed frog mesenteric capillaries analyzed by ultrathin serial sectioning.J. Elec. Micro. Techniq. 19:291–304, 1991.
Fu, B. M., R. Tsay, F. E. Curry, and S. Weinbaum. A junction-orifice-entrance layer model for capillary permeability: Application to frog mesenteric capillaries.ASME J. Biomech. Eng. 116:502–513, 1994.
Fu, B. M., F. E. Curry, and S. Weinbaum. A diffusion wake model for tracer ultrastructure-permeability studies in microvessels.Am. J. Physiol. 269:H2124-H2140, 1995.
Ganatos, P., S. Weinbaum, J. Fischbarg, and L. Liebovitch. A hydrodynamic theory for determining the membrane coefficients for the passage of spherical molecules through an intercellular cleft.Adv. Bioeng. 3:193–196, 1981.
Ghitescu, L., and M. Bendayan. Transendothelial transport of serum albumin: A quantitative immunocytochemical study.J. Cell Biol. 117:745–755, 1992.
Hewett, T. A., and S. Weinbaum. Generalized Oseen theory for heat and mass transfer: Flow past semi-infinite bodies.Phys. Fluids 18:119–127, 1975.
Kedem, O. and A. Katchalsky. Permeability of composite membranes.Trans. Faraday Soc. 59:1941–1953, 1963.
Mauri, R. Lagrangian self-diffusion of Brownian particles in periodic flow fields.Phys. Fluids 7:275–284, 1995.
Michel, C. C. Capillary permeability and how it may change.J. Physiol. 404:1–29, 1988.
Ogawa, K., T. Watabe, and K. Taniguchi. Transport pathways for macromolecules in the aortic endothelium: I. Transendothelial channels revealed by three-dimensional reconstruction using serial sections. Anat. Rec. 236:653–663, 1993.
Predescu, D., and G. E. Palade. Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium.Am. J. Physiol. 265:H725-H733, 1993.
Safford, R. E., E. A. Bassingthwaighte, and J. B. Bassingthwaighte. Diffusion of water in cat ventricular myocardium.J. Gen. Physiol. 72:513–538, 1978.
Schulze, C., and J. A. Firth. The interendothelial junction in myocardial capillaries: Evidence for the existence of regularly spaced, cleft-spanning structures.J. Cell Sci. 101:647–655, 1992.
Schneeberger, E. E., and M. Hamelin. Interaction of serum proteins with lung endothelial glycocalyx: Its effect on endothelial permeability.Am. J. Physiol. 247:H206-H217, 1984.
Schnitzer, J. E. Update on the cellular and molecular basis of capillary permeability.Trends. Cardiovasc. Med. 3:124–130, 1993.
Simionescu, M., and N. Simionescu. Ultrastructure of the microvascular wall: Functional correlations. In:Handbook of Physiology, edited by E. M. Renkin and C. C. Michel. Washington, DC: American Physiological Society, 1984, pp. 41–101.
Tsay, R., and S. Weinbaum. Viscous flow in a channel with periodic cross-bridging fibers of arbitrary aspect ratio and spacing.J. Fluid Mech. 226:125–148, 1991.
Turner, M. R., G. Clough, and C. C. Michel. The effects of cationised ferritin and native ferritin upon the filtration coefficient of single frog capillaries. Evidence that proteins in the endothelial cell coat influence permeability.Microvasc. Res. 25:205–222, 1983.
Wagner, R. C., and S. C. Chen. Transcapillary transport of solute by the endothelial vesicular system: Evidence from thin serial section analysis.Microvasc. Res. 42:139–150, 1991.
Weinbaum, S., P. Ganatos, R. Pfeffer, G. B. Wen, M. Lee, and S. Chien. On the time-dependent diffusion of macromolecules through transient open junctions and their subendothelial spread: I. Short-time model for cleft exit region.J. Theor. Biol. 135:1–30, 1988.
Weinbaum, S., R. Tsay, and F. E. Curry. A three-dimensional junction-pore-matrix model for capillary permeability.Microvasc. Res. 44:85–111, 1992.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fu, B., Curry, FR.E., Adamson, R.H. et al. A model for interpreting the tracer labeling of interendothelial clefts. Ann Biomed Eng 25, 375–397 (1997). https://doi.org/10.1007/BF02648050
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02648050