Abstract
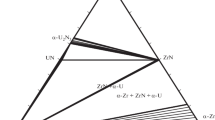
The relationships among the grand potential,G, the chemical potential, μi, and the effective chemical potential, μi, are geometrically demonstrated. An important equation concerning the relationship between the effective chemical potential, μi, and the molar free energy, Fm, was deduced. Use of the grand potential method enables calculation of the phase equilibria for the miscibility gap and different crystal structures in the ternary system.
Similar content being viewed by others
Cited References
R. Kikuchi,Acta Metall., 25,195 (1977).
R. Kikuchi,J. Chem. Phys., 60,1071 (1974).
S.M. Hao and M. Jing,Acta Metall. Sin., 28, B482 (1992).
M. Hillert,Thermodynamics and Diffusion in Alloys (in China), Metallurgical Industry Press, Beijing, People’s Republic of China (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shiming, H., Xin, Z. Calculation of phase equilibria for the ternary system by the grand potential method. JPE 16, 441–446 (1995). https://doi.org/10.1007/BF02645351
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02645351