Abstract
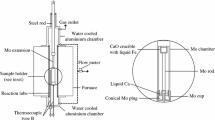
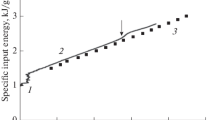
A constant volume technique was used to measure the rate of absorption of oxygen by liquid iron. The absorption process is found to proceed in two stages. When pure oxygen comes into contact with the surface of molten iron, an extremely rapid disappearance of oxygen from the gas phase accompanied by strong local super-heating of the melt at the gas/metal interface is observed. The rate of oxygen uptake during this stage is found to be dependent upon oxygen pressure and gas/metal interfacial area, but essentially independent of temperature, volume, and stirring conditions of the melt. The second phase commences after a few seconds with simultaneous cooling of the melt surface and the appearance at the gasmetal interface of a distinct third phase. The rate of absorption of oxygen during the second stage is markedly lower than the first stage. The presence of dissolved oxygen in the liquid iron has no influence on the absorption kinetics during either stage. A theoretical interpretation of the second stage is presented. A mechanism is proposed involving dissociative adsorption of oxygen molecules at the oxide/gas interface.
Similar content being viewed by others
References
N. S. Fortunatov and V. I. Mikhailovskaya:Memoirs of the Inst. of Chemistry, Acad. Sci. Ukrain S. S. R., 1940, vol. 6, pp. 83–92.
W. Lange:Zeits. Metallkunde, 1938, vol. 30, pp. 274–276.
G. Nandori:Kohaszati Lapok, 1957, vol. 12, pp. 49–53.
H. Hauttmann:J. Iron and Steel Inst. (London), 1961, vol. 198, pp. 410–11.
A. L. Veis and A. I. Rozlouski:Zhur. Fiz. Khim., 1949, vol. 23,pp. 1305–1310.
K. Teskes:Schwessen and Schneiden, 1956, No. 4, pp. 122–129.
D. G. C. Robertson and A. E. Jenkins:Heterogeneous Kinetics at Elevated Temperatures, G. R. Belton and W. L. Worrell, eds., pp. 393–408, Plenum Press, New York, 1970.
S. K. Vig and W-K Lu:J. Iron and Steel Inst. (London), 1971, vol. 209, pp. 630–634.
W.M. Boorstein and R. D. Pehlke:Trans. TMS-AIME, 1969, vol. 245,pp. 1843–1856.
Per Kofstad:High-Temperature Oxidation of Metals, p. 244, Wiley, New York, 1966.
Ibid.:High-Temperature Oxidation of Metals, Wiley, New York, 1966, pp. 245–46.
E. S. Machlin:Trans. TMS-AIME, 1960, vol. 218, pp. 314–26.
H. Knüppel and F. Oeters:Arch. Eisenhüttenw., 1962, vol. 33, pp. 729–36.
W. M. Boorstein: Ph.D. Thesis, Dept. of Chem. and Met. Engineering, The University of Michigan, 1967, p. 153.
K. E. Ōberg, L. M. Freidman, R. Szwarc, W. M. Boorstein and R. A. Rapp:J. Iron Steel Inst., 1972, vol. 210, pp. 359–62.
K. Susuki and K. Mori:Tetsu-to-Hagane, 1971, vol. 57, pp. 2219–29.
K. Schwerdtfeger:Trans. TMS-AIME, 1967, vol. 239, pp. 134–38.
A. McLean and R. G. Ward:J. Iron Steel Inst. 1966, vol. 204, pp. 8–11.
K. Hauffe:Sintering and Related Phenomena, G.C.Kuczynski, N. A. Hooton and C. F. Gibbon, eds., p. 139, Gordon and Breach, New York, 1967.
T. P. Hoar and L. E. Price:Trans. Faraday Soc, 1938, vol. 34, p. 867.
H. Inoue, J. W. Tmlinson and J. Chipman:Trans. Faraday Soc, 1953, vol. 49, pp. 796–801.
E. A. Pastihov, O. A. Esin and S. K. Churchumarev:Electrokhimiya, 1966, vol. 6, p. 209.
S. Takeuchi and K. Furukawa:Science Reports of the Research Institute, Tohoku Univ., Series A, 12, pp. 137–149, 1960.
L. S. Darken and R. W. Gurry:J. Amer. Chem. Soc, 1946, vol.68,pp.798–816.
P. Grieveson and E. T. Turkdogan:Trans. TMS-AIME, 1964, vol. 230, pp. 1609–14.
K. Mori and K. Suzuki:Trans. Iron and Steel Inst. Japan, 1969, vol. 9, pp. 409–12.
K. Mori and K. Suzuki:Tetsu-to-Hagane, 1968, vol. 54, pp. 1123–27.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Emi, T., Boorstein, W.M. & Pehlke, R.D. Absorption of gaseous oxygen by liquid iron. Metall Trans 5, 1959–1966 (1974). https://doi.org/10.1007/BF02644486
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02644486