Abstract
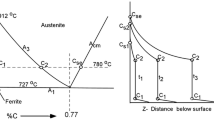
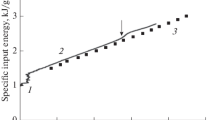
The rates of dissolution of solid pure iron and a solid iron-carbon alloy into molten iron-carbon alloys were studied “isothermally” as a function of temperature, carbon content and fluid dynamic (stirring) conditions. Experiments were carried out in a resistance-heated tube furnace under an inert atmosphere of argon gas at temperatures ranging from 2165 to 2563°F. A cylindrical specimen of the solid was preheated to the liquid temperature, immersed in the liquid bath contained in a graphite or alumina crucible, and rotated at speeds ranging up to 1800 rpm. The diameter of the specimen after partial solution was determined by direct measurement or calculated from the weight loss of the specimen. The experimental data were fitted with nondimensional correlations of mass transfer for a vertical cylinder, both stationary and rotating. When the specimen was stationary the mass transfer was dominated by natural convection in the liquid bath. When the specimen was rotated, forced convection prevailed and controlled the mass transfer rate.
Similar content being viewed by others
References
R. D. Pehlke,P. D. Goodell, and R. W. Dunlap:Trans. TMS-AIME, 1965, vol. 233, p. 1420.
R. G. Olsson, V. Koump, and T. F. Perzak:Trans. TMS-AIME, 1965, vol. 233, p. 1654.
M Kosaka and S. Minowa:Tetsu-to-Hagane, 1967, vol. 53, p. 983.
H. Nomura and K. Mori:Tetsu-to-Hagane, 1969, vol. 55, p. 1134.
R. I. L. Guthrie and P. Stubbs: "Kinetics of Slag Melting in Quiescent Baths of Molten Pig Iron," presented at AIME Annual Meeting, Chicago, Feb. 1973.
G. M. Glinkov,et ai. Steel USSR,March 1971, pp. 201–3.
G. M. Glinkov,et al. II. Vyssh. Uchet. Zaved. Chern. Met., 1972, pp. 62–64.
J. Szekely, Y. K. Chuang, and J. W. Hlinka:Met. Trans., 1972, vol. 3, p. 2825.
J. M. Lommel and B. Chalmers:Trans. TSM-AIME, 1959, vol. 215, p. 499.
J. H. Perry:Chemical Engineers’ Handbook, 4th Ed., p. 10–10, McGraw-Hill, New York, 1963.
J. Szekely and N. J. Themelis:Rate Phenomena in Process Metallurgy, p. 457, Wiley, New York, 1971.
M. Eisenberg, C. W. Tobias, and C. R. Wilke:Chem. Eng. Prog. Symp. Series, No. 16,1955, vol. 51, p. 1
Yeong-U Kim:Ph.D. Thesis, The University of Michigan, Ann Arbor, Michi- gan, 1973.
J. F. Elliott, M. Gleiser, and V. Ramakrishma:Thermochemistry for Steel- making, Vol. II, p. 349, Addison-Wesley, 1963.
V. C. Benedicks, N. Ericsson, and G. Ericson:Arch. Eisenhüttenw., 1930, vol. 3, p. 473.
B. P. Burylev:Russ. J. Phys. Chem., 1967, vol. 41, p. 53.
M. Kosaka and S. Minowa:Tetsu-to-Hagane, 1966, vol. 52, p. 1748.
H. Schlichting:Boundary Layer Theory, p. 439, McGraw-Hill, New York, 1960.
Author information
Authors and Affiliations
Additional information
This paper is based in part on a thesis submitted by Y-U. Kim in partial fulfillment of the requirements for the degree Doctor of Philosophy at the University of Michigan.
Rights and permissions
About this article
Cite this article
Kim, YU., Pehlke, R. Mass transfer during dissolution of a solid into liquid in the iron-carbon system. Metall Trans 5, 2527–2532 (1974). https://doi.org/10.1007/BF02643873
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02643873