Abstract
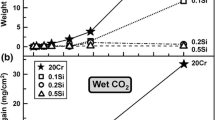
This report deals with the kinetics and mechanism of the reaction of iron-chromium and iron-chromium-molybdenum alloys (containing 1.25 pct Cr-0.5 pct Mo, 5 pct Cr-0.5 pct Mo, and 12 pct Cr) with low partial pressure chlorine gas in the temperature range 270 to 550 °C. The rate of reaction generally decreases with exposure time and, in the case of alloys containing five pct Cr and higher chromium content, the kinetics follows a parabolic rate law. The reaction kinetics is influenced by surface films containing FeCl2 and CrCl3 which have very low vapor pressure in the temperature range studied. The effect of molybdenum on the reaction is negligible. In the case of alloys containing five pct Cr-0.5 pct Mo and 12 pct Cr, the reaction product film consists essentially of CrCl3 (outer layer) and much smaller concentration of FeCl2 (inner layer). For these alloys, the observed parabolic kinetics is attributed to kinetic control of the overall reaction by solid-state diffusion through the reaction product surface film. In the case of the 1.25 pct Cr-0.5 pct Mo alloy, the film consists essentially of a mixture of FeCl2 and CrCl3. In this case, an outer CrCl3 film layer is not formed and the overall kinetics is influenced by the rate of formation of volatile FeCl3 speciesvia the reaction 2 FeCl2 + Cl2 (FeCl3)2.
Similar content being viewed by others
References
K. L. Tseitlin and V. A. Strumkin:Zh. Fiz. Khim., 1958, vol. 30, pp. 1843–51.
M. H. Brown, W. B. DeLong, and J. R. Auld:Ind. Eng. Chem., 1947, vol. 39, pp. 839–44.
The Chemistry and Metallurgy of Miscellaneous Materials: Thermo-dynamics, L. L. Quill, ed., McGraw-Hill Book Company, Inc., New York, NY, 1950, pp. 193–204.
A. Glassner: “The Thermochemical Properties of Oxides, Fluoride and Chlorides to 2500 K”, Report ANL-5750, Argonne National Laboratory.
R. F. Fruehan:Metall. Trans., 1972, vol. 3, pp. 2585–92.
Z. A. Foroulis: inMetal-Slag-Gas Reactions and Processes, Z. A. Foroulis and W. W. Smeltzer, eds.,The Electr. Soc, Inc., Princeton, NJ, 1975, pp. 571–80.
Z. A. Foroulis:Werkstoffe u. Korrosion, in press.
Z. A. Foroulis:J. Electr. Soc, 1981, vol. 128, pp. 487–89.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Foroulis, Z.A. Kinetics and mechanism of the reaction of iron-chromium and iron-chromium-molybdenum alloys with chlorine gas. Metall Trans A 13, 153–159 (1982). https://doi.org/10.1007/BF02642427
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02642427