Summary
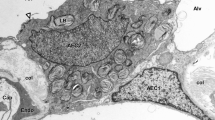
Considerable progress has recently been made in the understanding of airway inflammation by cell culture assays and in vivo provocation studies. Inasmuch as ethical considerations limit experimental work in humans, physiologically relevant in vitro models are required to better understand cellular and molecular tissue interactions in human nasal mucosa. Here we describe a human nasal mucosa culture model utilizing a simple gelatin sponge-supported histoculture system at the air-liquid interface. Viable mucosa was preserved for at least 48 h, as shown by morphology and immunohistochemical staining with Ki-67 as marker for proliferation. Pro-inflammatory mediators (kinins, histamine, thromboxane B2, prostaglandin F2α, and substance P) are detectable in serum-containing as well as serum-free culture medium. Incubation with 10−8 M substance P increases the number of degranulated mast cells after 48 h by 26% (P<0.01). In this model, biochemical responses can be correlated with histologic alterations of the target tissue. Inflammatory parameters can be examined and compared in various patient groups and different stimulators/inhibitors. This culture method provides a valuable research tool for analyzing all compartments present in nasal mucosa under physiologically relevant conditions, and for studying complex interactions and responses of mucosal cell populations in their natural tissue environment.
Similar content being viewed by others
References
Barnes, P. J. Asthma as an axon reflex. Lancet 1:242–245; 1986.
Baumgarten, C. R.; Lehmkuhl, B.; Henning, R., et al. Bradykinin and other inflammatory mediators in BAL-fluid from patients with active pulmonary inflammation. In: Bönner, G.; Fritz, H.; Schoelkens, B., eds. Recent progress on kinins, vol. 38/III. Basel: Birkhäuser; 1992:475–481.
Bradding, P.; Feather, I. H.; Wilson, S., et al. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J. Immunol. 151:3853–3865; 1993.
Cordell, J.; Falini, B.; Erber, O. N., et al. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J. Histochem. Cytochem. 32:219–227; 1984.
Devalia, J. L.; Davies, R. J. Airway epithelial cells and mediators of inflammation. Editorial. Respir. Med. 87:405–408; 1993.
Devalia, J. L.; Sapsford, R. J.; Wells, C., et al. Culture and comparison of human bronchial and nasal epithelial cells in vitro. Respir. Med. 84:303–312; 1990.
Ebertz, J. M.; Hirshman, C. A.; Kettelkamp, N. S. Substance P induced histamine release in human cutaneous mast cells. J. Invest. Dermatol. 88:682–685; 1987.
Gerdes, J.; Schwab, U.; Lemke, H., et al. Production of mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Cancer 31:13–20; 1983.
Hoffman, R. M. Three-dimensional histoculture: origin and applications in cancer research. Cancer Cells 3:86–92; 1991.
Leighton, J. A sponge matrix method for tissue culture. JNCI 12:545–561; 1951.
Li, L.; Margolis, L. B.; Paus, R., et al. Hair shaft elongation, follicle growth and spontaneous regression in long-term, sponge-gel supported histoculture of human scalp skin. Proc. Natl. Acad. Sci. USA 89:8764–8768; 1992.
Li, L.; Paus, R.; Slominski, A., et al. Skin histoculture assay for studying the hair cycle. In Vitro Cell. Dev. Biol. 28A:695–698; 1992.
Liu, M. C.; Hubbard, W. C.; Proud, D., et al. Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Am. Rev. Respir. Dis. 144:51–58; 1991.
Nieber, K.; Baumgarten, C. R.; Rathsack, R., et al. Substance P andβ-endorphine-like immunoreactivity in lavage fluids of subjects with and without allergic asthma. J. All Clin. Immunol. 90:646–652; 1992.
Proud, D.; Togias, A.; Naclerio, R. M., et al. Kinins are generated in vivo following nasal airway challenge of allergic individuals with allergen. J. Clin. Invest. 72:1678; 1983.
Siraganian, R. An automated continuous flow system for the extraction and fluometric analysis of histamine. Anal. Biochem. 57:283–294; 1974.
Zhang, M.; Niehus, J.; Schnellbacher, T., et al. ELISA for the neuropeptide degrading endopeptidase 3.4.24.11 in human serum and leukocytes. Peptides 15:843–848; 1994.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schierhorn, K., Brunnée, T., Paus, R. et al. Gelatin sponge-supported histoculture of human nasal mucosa. In Vitro Cell Dev Biol - Animal 31, 215–220 (1995). https://doi.org/10.1007/BF02639436
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02639436