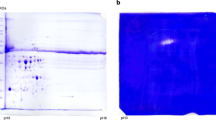
Abstract
The normal development of tracheary elements (TE) requires a selective degradation of the cytoplasm without loss of the extracellular wall that remains behind as the water-conducting units of xylem. Using zinnia-(Zinnia elegans L. cv. Green Envy) cultured mesophyll cells that synchronously transdifferentiate into TEs, extracellular and intracellular proteases, respectively, have been shown to both trigger death and to execute autolysis as the final component of a programmed cell death (PCD). We report here the appearance in the medium of an unusual proteolytic activity correlated with the PCD process just prior to the autolysis. The activity has a pH optimum of 5.5–6.0 and displays some thrombin characteristics. This protease activity has 1) a 10-fold higher affinity towards a thrombin-specific chromogenic substrate than toward a trypsin-specific chromogenic substrate; 2) a 1000-fold lower sensitivity to soybean trypsin inhibitor (STI) compared to trypsin; and 3) limited ability to cleave the protease-activated receptor-1, the native thrombin substrate. However, the addition of partially purified fraction containing the thrombin-like protease activity to the medium of PCD-competent cells does not prematurely trigger PCD, and the thrombin-specific peptide inhibitor phenylalanine-proline-aspartic acid-chloromethylketone fails to inhibit PCD or tracheary element (TE) formation. This suggests that this protease activity may play a role within the cells in execution of the autolysis or in the collapse of the tonoplast rather than as an extracellular proteolytic activity participating in the chain of events leading to cell death.
Similar content being viewed by others
References
Beers EP, Freeman TB. 1997. Proteinase activity during tracheary element differentiation in zinnia mesophyll cultures. Plant Physiol 113:873–880.
Beers EP, Jones AM, Dickerman A. 2004. The S8 serine, C1A cysteine, and A1 aspartic protease families in Arabidopsis Phytochemistry 651:43–58.
Burgess J, Linstead P. 1984.In-vitro tracheary element formation: structural studies and the effect of tri-iodobenzonic acid. Planta 160:481–489.
Callis J. 1995. Regulation of protein degradation Plant Cell 7:845–857.
Cappiello M, Vilardo PG, Lippi A, Criscuoli M, Corso A Del, Mura U. 1996. Kinetics of human thrombin inhibition by two novel peptide inhibitors (Hirunorm IV and Hirunorm V). Biochem Pharmacol 52:1141–1146.
Fioretti E, Angeletti M, Fiorucci L, Barra D, Bossa F, Ascoli F. 1988. Aprotinin-like isoinhibitors in bovine organs. Biol Chem Hoppe Seyler 369. Suppl:37–42.
Fukuda H. 1992. Tracheary element formation as a model system of cell differentiation. Int Rev Cytol 136:289–332.
Fukuda H, Komamine A. 1980. Establishment of an experimental system for the study of tracheary element differentiation from single cells isolated from the mesophyll ofZinnia elegans Plant Physiol 65:57–60.
Groover A, Dewitt N, Heidel A, Jones AM 1997. Programmed cell death of plant tracheary elements differentiatingin vitro. Protoplasma 196:197–211.
Groover A, Jones AM. 1999. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol 119:375–384.
Hung DT, Vu TH, Nelken NA, Coughlin SR. 1992. Thrombin-induced events in non-platelet cells are mediated by the unique proteolytic mechanism established for the cloned platelet thrombin receptor. J Cell Biol 116:827–832.
Ishii K, Hein L, Kobilka B, Coughlin SR. 1993. Kinetics of thrombin receptor cleavage on intact cell. J Biol Chem 268:9780–9786.
Ito J, Fukuda H. 2002. ZENI is a key enzyme in the degradation of nuclear DNA during programmed cell death of Tracheary Elements Plant Cell 14:3201–3211.
Kuriyama H. 1999. Loss of tonoplast integrity programmed in tracheary element differentiation Plant Physiol 121:763–774.
Markwardt F, Walsmann P. 1967. Purification and analysis of the thrombin inhibitor hirudin. Hope Seylers Z Physiol Chem 348:1381–1386.
Matsuura K, Yamamoto K, Sinohara H. 1994. Amidase activity of human Bence Jones proteins Biochem Biophys Res Commun 204:57–62.
Obara K, Kuriyama H, Fukuda H. 2001. Direct evidence of active and rapid nuclear degradation triggered by vacuole rupture during programmed cell death in zinnia. Plant Physiol 125:615–626.
Rahr HB, Sorensen JV, Danielsen D. 1994. Markers of coagulation and fibrinolysis in blood drawn into citrate with and without D-Phe-Pro-Arg-chloromethylketone (PPACK) Thromb Res 73:279–284.
Smirnova IV, Zhang SX, Citron BA, Arnold PM, Festoff BW. 1998. Thrombin is an extracellular signal that activates intracellular death protease pathways inducing apoptosis in model motor neurons. J Neurobiol 36:64–80.
Trejo A, Coughlin SR. 1999. The cytoplasmic tails of protease-activated receptor-1 and substance P receptor specify sorting to lysosomeversus recycling. J Biol Chem 274:2216–2224.
Trejo J, Altschuler Y, Fu HW, Mostov KE, Coughlin SR. 2000. Protease-activated receptor-1 down-regulation: a mutant HeLa cell line suggests novel requirements for PAR1 phosphorylation and recruitment to clathrin-coated pits. J Biol Chem 275:31255–31265.
Uren AG, O'Rourke K, Aravind L, et al. 2000. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT Lymphona Molecular Cell 6:961–967.
Vu TK, Hung DT, Wheaton VI, Coughlin SR. 1991. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 64:1057–1068.
Williams EB, Krishnaswamy S, Mann KG. 1989. Zymogen/enzyme discrimination using peptide chloromethyl ketones. J Biol Chem 264:7536–7545.
Wunderwald P, Schrenk WJ, Port H. 1982. Antithrombin BM from human plasma: an antithrombin binding moderately to heparin. Thromb Res 25:177–191.
Ye Z-H, Varner JE. 1996. Induction of cysteine and serine proteinases during xylogenesis inZinnia elegans. Plant Mol Biol 30:1233–1246.
Yu X-H, Perdue TD, Heimer YM, Jones AM. 2002. Mitochondrial involvement in tracheary element programmed cell death. Cell Death Differ 9:189–198.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Online publication: 7 April 2005
Rights and permissions
About this article
Cite this article
Yu, XH., Jones, B., Jones, A.M. et al. A protease activity displaying some thrombin-like characteristics in conditioned medium of zinnia mesophyll cells undergoing tracheary element differentiation. J Plant Growth Regul 23, 292–300 (2004). https://doi.org/10.1007/BF02637252
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02637252