Abstract
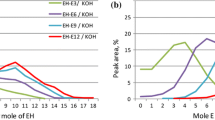
Polyglycol nonionic surfactants are widely used in industrial and consumer products. Two classes of these surfactants, made from selected combinations of 1,2-butylene oxide, propylene oxide and ethylene oxide, were compared to alcohol ethoxylate (AE) and nonyl phenol ethoxylate nonionic surfactants in this study. Polyglycol copolymers consisted of either a polypropylene glycol (PPG) or polybutylene glycol (PBG) central hydrophobe. Ethoxylation of the hydrophobes produced polyethylene glycol hydrophilic blocks. Differences in hydrophobe polarity were determined by inverse gas chromatography (IGC). IGC is a useful analytical method by which the physical and chemical characteristics of a material are studied. The stationary surfactant material under study was coated onto an inert support and used as the packing for the column. A probe mixture, containing simple organic molecules of varying polarity, was injected, and the retention characteristics were measured. The retention characteristics of the standard probe mixture were used to reveal relative polarity information about the stationary surfactant coatings. Polarities of the four hydrophobes were (in decreasing order): PPG, PBG, nonyl phenyl and fatty alkyl. Comparisons were then made between the calculated hydrophile-lipophile balance values and polarity indices of the hydrophobes and their ethoxylates. The effects of hydroxyl groups on polarity were also studied and quantified.
Similar content being viewed by others
References
Becher, P., and R.L. Birkmeier,J. Am. Oil Chem. Soc. 41:169 (1964).
Munk, P., inModern Methods of Polymer Characterization, edited by H.G. Barth, and J. W Mays, John Wiley and Sons, New York, 1991, Chapter 5, p. 151.
Newman, R.D., and J.M. Prausnitz,J. Paint Tech. 45:33 (1973).
Schott, H.,J. Pharm. Sci. 73:790 (1984).
Sen, A.K., and R. Kumar,J. Soc. Trib. Lub. Eng. 47:211 (1991).
Poole, C.F., T.O. Kollie and S.K. Poole,Chromatographia 34:281 (1992).
Kersten, B.R., and C.F. Poole,J. Chromatog. 452:191 (1988).
Inui, T., Y. Murakami, T. Suzuki and Y. Takegami,Polymer J. 14:261 (1982).
Munk, P., Z.Y. Al-Saigh and T.W. Card,Macromolecules 18:2196 (1985).
Kollie, T.O., and C.F. Poole,J. Chromatog. 556:457 (1991).
Heubner, V.R.,Analytical Chem. 34:488 (1962).
Miller, S.A., B. Bann and R.D. Thrower,J. Chem. Soc. 3623 (1950).
Szymanowski, J., J. Nowicki and A. Voelkel,Coll. & Pol. Sci. 257:494 (1979).
Voelkel, A., J. Szymanowski and W. Hreczuch,J. Am. Oil Chem. Soc. 70:711 (1993).
Author information
Authors and Affiliations
About this article
Cite this article
Nace, V.M., Knoell, J.C. Nonionic surfactant polarity index determination by inverse gas chromatography. J Am Oil Chem Soc 72, 89–95 (1995). https://doi.org/10.1007/BF02635784
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02635784