Summary
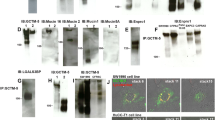
A mammalian embryonic cell surface glycoprotein (ESGp), whose expression and biochemical structure seem to be developmentally regulated, has been isolated and characterized. The molecule expressed in two cell through morula stage mouse embryos has a molecular weight, by electrophoretic analyses, of 90 kDa. At the blastocyst stage, however, the molecule migrates as a broad, heterogeneous band ranging from 90 to 110 kDa. Evidence obtained from studies of embryonal carcinoma (EC) cells indicates that this band is actually a composite of three distinct molecules (molecular weight 90, 95, and 105 to 110 kDa), each of which is synthesized uniquely by one of the different cell types of the blastocyst: the embryonic ectoderm and visceral and parietal endoderms, respectively. A survey of various mouse tissues and cell lines has revealed that undifferentiated cells express the low molecular weight form (90 kDa) characteristic of embryonic ectoderm, whereas differentiated cells and adult tissues express the high molecular weight form (110 kDa) characteristic of parietal endoderm. Only the EC visceral endoderm cell analogues have been shown to express the intermediate molecule (95 kDa). In embryos, the antigen is uniformly distributed over the cell surface during early cleavage stages (two to eight cell); just before compaction, however, it seems to redistribute and becomes polarized at the outside exposed edges of blastomeres. In cultured EC cells, ESGp is found only in areas of cell-to-cell contact; free-standing surfaces of cells are negative for expression. It is possible, therefore, that ESGp may be involved in the intercellular adhesion of both EC cells and compacting embryos.
Similar content being viewed by others
References
Adamson, E. D.; Evans, M. S.; Magrane, G. G. Biochemical markers of the progress of differentiation in cloned teratocarcinoma cell lines. Eur. J. Biochem. 79:607–615; 1977.
Bernstine, E. C.; Hooper, N. L.; Grandchamp, S., et al. Alkaline phosphatase activity in mouse teratoma. Proc. Natl. Acad. Sci. USA 70:3899–3903; 1973.
Cheng, C. C.; Sege, K.; Alton, A. K., et al. Characterization of an antigen present on testicular cells and preimplantation embryos whose expression is modified by t12 haplotype. J. Immunogenet. 10:465–485; 1983.
Cossu, G.; Warren, L. Lactosaminoglycans and heparin sulfate are covalently bound to fibronectins synthesized by mouse stem teratocarcinoma cells. J. Biol. Chem. 258:5603–5607; 1983.
D’Eustachio, P.; Owens, G.; Edelman, G. M., et al. Chromosomal location of the gene encoding the neural cell adhesion molecule (N-CAM) in the mouse. Proc. Natl. Acad. Sci. USA 82:7631–7635; 1988.
Damsky, C. H.; Richa, J.; Solter, D., et al. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell 34:455–466; 1983.
Dustin, M. L.; Rothlein, R.; Bhan, A. K., et al. Induction by IL-1 and interferon, tissue distribution, biochemistry and function of a natural adherence molecule (ICAM-1). J. Immunol. 137:245–254; 1986.
Edelman, G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Ann. Rev. Cell Biol. 2:81–116; 1986.
Edelman, G. M.; Gallin, W. J.; Delouvee, A., et al. Early epochal maps of two different cell adhesion molecules. Proc. Natl. Acad. Sci. USA 80:4384–4388; 1983.
Edwards, R. G.; Gates, A. H. Timing of the stages of the maturation divisions, ovulation, fertilization, and the first cleavage of eggs of adult mice treated with gonadotrophins. J. Endocrinol. 18:292–304; 1959.
Friedlander, D. R.; Brackenbury, R.; Edelman, G. M. Conversion of embryonic forms of N-CAMin vitro results fromde novo synthesis of adult forms. J. Cell Biol. 101:412–419; 1985.
Hyafil, F.; Babinet, C.; Jacob, F. Cell-cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell 26:447–454; 1981.
Hyafil, F.; Morello, D.; Babinet, C., et al. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell 21:927–934; 1980.
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277:680–685; 1970.
Lehman, J. M.; Speers, W. C.; Swartzendrubner, D. E., et al. Neoplastic differentiation: characteristics of cell lines derived from a murine teratocarcinoma. J. Cell. Physiol. 84:13–28; 1974.
Marlin, S. D.; Springer, T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell 51:81–116; 1987.
Martin, G. R. Teratocarcinomas and mammalian embryogenesis. Science 209:768–776; 1980.
Martin, G. R.; Evans, M. J. The formation of embryoid bodies in vitro by homogeneous embryonal carcinoma cell cultures derived from isolated single cells. In: Sherman, M. I.; Solter, D., eds. Teratomas and differentiation. New York: Academic Press; 1975:169–187.
McCormick, P. J.; Babiarz, B. Expression of a glucose-regulated cell surface protein in early mouse embryos. Dev. Biol. 105:530–534; 1984.
McCormick, P. J.; Millis, A. J. T.; Babiarz, B. Distribution of a 100K dalton glucose-regulated cell surface protein in mammalian cell cultures and sectioned tissues. Exp. Cell Res. 138:63–72; 1982.
McCormick, P. J.; Dimeo, A.; Neuner, E., et al. Characterization of the F9 antigen(s) isolated from teratocarcinoma cell culture medium. Cell Differ. 11:135–140; 1982.
McCormick, P. J.; Shin, H.-S. Analysis of a nontumorigenic embryonal carcinoma cell line. Exp. Cell. Res. 189:183–188; 1990.
Muramatsu, T.; Gachelin, G.; Jacob, F. Characterization of glycopeptides isolated from membranes of F9 embryonal carcinoma cells. Biochim. Biophys. Acta 587:392–406; 1979.
Muramatsu, T.; Gachelin, G.; Nicolas, J. F., et al. Carbohydrate structure and cell differentiation: unique properties of fucosyl-glycopeptides isolated from embryonal carcinoma cells. Proc. Natl. Acad. Sci. USA 75:2315–2319; 1978.
Sato, M.; Muramatsu, T.; Berger, E. G. Immunological detection of cell surface galactosyltransferase in preimplantation mouse embryos. Dev. Biol. 102:514–518; 1984.
Shirayoshi, Y.; Hatta, K.; Hosoda, M., et al. Cadherin cell adhesion molecules with distinct binding specificities share a common structure. EMBO J. 5:2485–2488; 1986.
Shur, B. D. Embryonal carcinoma cell adhesion: the role of surface galactosyltransferase and its 90 k lactosaminoglycan substrate. Dev. Biol. 99:360–372; 1983.
Simmons, D.; Makgoba, M. W.; Seed, B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature 331:624–627; 1988.
Solter, D.; Knowles, B. B. Monoclonal antibody defining a stage specific mouse embryonic antigen (SSEA-1). Proc. Natl. Acad. Sci. USA 75:5565–5569; 1978.
Strickland, S. Mouse teratocarcinoma cells: prospects for the study of embryogenesis and neoplasia. Cell 24:277–278; 1981.
Strickland, S.; Mahdavi, V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell 15:393–403; 1978.
Takeichi, M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development 102:639–655; 1988.
Yoshida-Noro, C.; Suzuki, N.; Takeichi, M. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev. Biol. 101:19–37; 1984.
Author information
Authors and Affiliations
Additional information
This work was supported by grant R01 HD23402 from the National Institutes of Health, Bethesda, MD.
Rights and permissions
About this article
Cite this article
McCormick, P.J. Characterization of a developmentally regulated mouse embryonic antigen. In Vitro Cell Dev Biol - Animal 27, 260–266 (1991). https://doi.org/10.1007/BF02630927
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02630927