Summary
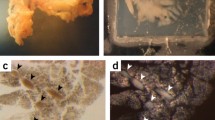
Interlobular duct fragments from the pancreas of the rat were isolated by collagenase digestion and filtration, embedded in a matrix of rat-tail collagen, and cultured in a 1∶1 mixture of Dulbecco’s minimal essential and Ham’s F12 media supplemented with cholera toxin (CT, 100 ng/ml) and epidermal growth factor (EGF, 10 ng/ml) in addition to supplements used previously, thereby improving the yield of ducts by a factor of two compared with previous resuts. The ducts were harvested by digestion of the collagen matrix with collagenase and were then dissociated by treatment with EDTA in divalent cation-free salt solution, followed by digestion with collagenase and hyaluronidase. The resulting tissue fragments were suspended in collagen and cultured as were the ducts. Numerous cysts appeared as a function of time and some of these enlarged dramatically. Some of the larger cysts exhibited secondary tubular processes extending into the surrounding collagen. The addition of bovine pituitary extract (BPE, 50 μg/ml) doubled the number of cysts, whereas omission of serum or CT+EGF reduced the number. BPE or forskolin could substitute effectively for CT. Agents that stimulate (secretin) or inhibit (e.g., ouabain or acetazolamide) fluid-electrolyte secretion in vivo had no effect on the number or average diameter of the cysts. The cysts were 83 to 88% epithelial with the balance of the cells being fibroblastic in appearance. Some cysts consisted only of epithelium. The proliferative capacity of the cystic epithelium was shown, by the presence of mitotic figures and by an autoradiographic labeling index of 22 to 30% after a 24-h exposure to [3H]thymidine. The labeling index was reduced by the omission of CT+EGF. Transmission electron microscopy showed that the cysts exhibited morphologic features of duct epithelium in vivo, including apical microvilli, lateral, interdigitations of the plasma membrane, and typical cytoplasmic organelles.
Similar content being viewed by others
References
Githens, S. The pancreatic duct cell: proliferative capabilities, specific characteristics, metaplasia, isolation and culture. J. Pediatr. Gastroenterol. Nutr. 7:486–506; 1988.
Case, R. M.; Argent, B. E. Bicarbonate secretion by pancreatic duct cells: mechanism, and control. In: Go, V. L. W.; Gardner J. D.; Brooks, F. P., et al. eds. The exocrine pancreas: biology, pathobiology, and diseases. New York: Raven Press; 1986:213–244.
Kuijpers, G. A. J.; dePont, J. J. H. Role of proton and bicarbonate transport in pancreatic cell function. Annu. Rev. Physiol 49:87–103; 1987.
Schulz, I. Electrolyte and fluid secretion in the exocrine pancreas. In: Johnson, L. R., ed. Physiology of the gastrointestinal tract, 2nd ed. New York: Raven Press; 1987:1147–1171.
Forstner, G.; Forstner, J. Mucus: biosynthesis and secretion. In: Go, V. L. W.; Gardner, J. D.; Brooks, F. P., et al., eds. The exocrine pancreas: biology, pathobiology, and diseases. New York: Raven Press; 1986:283–286.
Githens, S.; Holmquist, D. R. G.; Whelan, J. F., et al. Ducts of the rat pancreas in agarose matrix culture. In Vitro 16:797–808; 1980.
Githens, S.; Finley, J. J.; Patke, C. L., et al. Biochemical and histochemical characterization of cultured rat and hamster pancreatic ducts. Pancreas 2:427–438; 1987.
Arkle, S.; Lee, C. M.; Cullen, M. J., et al. Isolation of ducts from the pancreas of copper-deficient rats. Q. J. Exp. Physiol. 71:249–265; 1986.
Stoner, G. D.; Harris, C. C.; Bostwick, D. G., et al. Isolation and characterization of epithelial cells from bovine pancreatic duct. In Vitro 14:581–590; 1987.
Jones, R. T.; Trump, B. F.; Stoner, G. D. Culture of human pancreatic ducts. Methods Cell Biol. 21B:429–439; 1980.
Sato, T.; Sato, M.; Hudson, E. A., et al. Characterization of bovine pancreatic ductal cells isolated by a perfusion-digestion technique. In Vitro 19:651–660; 1983.
Hootman, S. R.; Logsdon, C. D. Isolation and monolayer culture of guinea pig pancreatic duct epithelial cells. In Vitro 24:566–574; 1988.
Tsao, M.-S.; Duguid, W. P. Establishment of propagable epithelial cell lines irom normal adult rat pancreas. Exp. Cell. Res. 168:365–375; 1987.
Githens, S.; Whelan, J. F. Isolation culture of hamster pancreatic ducts. J. Tissue Cult Methods 8:97–102; 1983.
Githens, S.; Patke, C. L.; Schexnayder, J. A. Culture of rat pancreatic interlobular duct epithelium in collagen gel. J. Cell Biol. 103:360a; 1986.
Richards, J.; Larson, L.; Yang, J., et al. Method for culturing mammary epithelial cells in a rat tail collagen gel matrix. J. Tissue Cult. Methods. 8:31–36; 1983.
Amsterdam, A.; Jamieson, J. D. Studies on dispersed pancreatic exocrine cells. I. Dissociation technique and morphologic characteristics of separated cells. J. Cell Biol. 63:1037–1056; 1974.
Oliver, C. Isolation and maintenance of differentiated exocrine gland acinar cells in vitro. In Vitro 16:297–305; 1980.
Karnovsky, M. J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 27:137A-138A; 1965.
Iacino, D.; Scheele, G. A.; Liebow, C. Secretory response of the rabbit pancreas to cholecystokinin stimulation. Am. J. Physiol. 239:G247-G254; 1980.
Smith, P. A.; Case, R. M. Effects of cholera toxin on cyclic adenosine 3′,5′-monophosphate concentration and secretory processes in the exocrine pancreas. Biochim. Biophys. Acta 399:277–290; 1975.
Harris, A.; Coleman, L. Establishment of a tissue culture system for epithelial cells derived from human pancreas: a model for the study of cystic fibrosis. J. Cell Sci. 87:695–703; 1987.
Chen, J.; Stuckey, E. C.; Berry, C. L. Three-dimensional culture of rat exocrine pancreatic cells using collagen gels. Br. J. Exp. Pathol. 66:551–559; 1985.
Mueller, H. B. Der Einfluss kupferarmer Kost auf das Pankreas. Lichtmikroskopische Untersuchungen am exokrinen Teil der Bauchspeicheldrusen weisser Ratten. Virchows Arch. [A] 350:353–367; 1970.
Argent, B. E.; Arkle, S.; Cullen, M. J., et al. Morphological, biochemical, and secretory studies on rat pancreatic ducts maintained in tissue culture. Q. J. Exp. Physiol. 71:633–648; 1986.
Resau, J. H.; Hudson, E. A.; Jones, R. T. Organ explant culture of adult Syrian golden hamster pancreas. In Vitro 19:315–325; 1983.
Kern, H. F.; Ferner, H. Die Feinstruktur der exokrinen Pancreasgewebes vom Menschen. Z. Zellforsch. Mikrosk. Anat. 113:322–343; 1971.
Riches, D. J. Ultrastructural observations on the common bile duct epithelium of the rat. J. Anat. 111:157–170; 1972.
Cohen, P. J. The renewal areas of the common bile epithelium in the rat. Anat. Rec. 150:237–242; 1964.
Kirk, D.; Alverez, R. B. Morphologically stable epithelial vesicles cultured from normal human endometrium in defined medium. In Vitro 22:604–614; 1986.
Yang, J.; Nandi, S. Growth of cultured cells using collagen as substrate. Int. Rev. Cytol. 81:249–286; 1983.
Iguchi, T.; Uchima, F.-D. A.; Bern, H. A. Growth of mouse vaginal epithelial cells in culture: effect of sera and supplemented serum-free media. In Vitro 23:535–540; 1987.
Sanderson, R. D.; Fitch, J. M.; Linsenmayer, T. R., et al. Fibroblasts promote the formation of a continuous basal lamina during myogenesis in vitro. J. Cell Biol. 102:740–747; 1986.
Peehl, D. M.; Stamey, T. A. Serum-free growth of adult human prostatic epithelial cells. In Vitro 22:82–90; 1986.
Ethier, S. P. Primary culture and serial, passage of normal and carcinogen-treated rat mammary epithelial cells in vitro. JNCI 71:1307–1318; 1985.
Carpenter, G.; Cohen, S. Epidermal growth factor. Ann. Rev. Biochem. 48:193–216; 1979.
Seamon, K. B.; Padgett, W.; Daly, J. W. Forskolin: unique diterpene activation of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. USA 78:3363–3367; 1981.
Kempen, H. J. M.; dePont, J. J. H.; Bonting, S. L. Rat pancreas adenylate cyclase. III. Its role in pancreatic secretion assessed by means of cholera toxin. Biochim. Biophys. Acta. 392:276–287; 1975.
Hoffman, E. K. Anion transport systems in the plasma membrane of vertebrate cells. Biochim. Biophys. Acta. 864:1–31; 1986.
Schulz, I. Bicarbonate transport in the exocrine pancreas. Ann. NY Acad. Sci. 341:191–209; 1980.
Tomooka, Y.; Harris, S. E. McLachlan, J. A. Growth of seminal vesicle epithelial cells in serum-free collagen gel culture. In Vitro 21:237–244; 1985.
Montesano, R.; Mouson, P.; Amherdt, M., et al. Collagen matrix promotes reorganization of pancreatic endocrine cell monolayers into islet-like organoids. J. Cell Biol. 97:935–939; 1983.
Logsdon, C. D.; Williams, J. A. Pancreatic acinar cells in monolayer culture: direct trophic effects of caerulein in vitro. Am. J. Physiol. 250:G440-G447; 1986.
Oliver, C.; Waters, J. F.; Tolbert, C. L., et al. Growth of exocrine acinar cells on a reconstituted basement membrane gel. In Vitro 23:465–473; 1987.
Bendayan, M.; Duhr, M. A.; Gingras, D. Studies on pancreatic acinar cells on tissue culture: basal lamina (basement membrane) matrix promotes three-dimensional reorganization. Eur. J. Cell Biol. 42:60–67; 1986.
Thivolet, C. H.; Chatelain, P.; Nicoloso, H., et al. Morphological and functional effects of extracellular matrix on pancreatic islet cell cultures. Exp. Cell Res. 159:313–322; 1985.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Githens, S., Schexnayder, J.A., Desai, K. et al. Rat pancreatic interlobular duct epithelium: Isolation and culture in collagen gel. In Vitro Cell Dev Biol 25, 679–688 (1989). https://doi.org/10.1007/BF02623720
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02623720