Summary
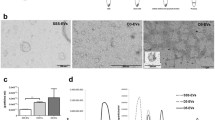
Epithelium from normal human endometrium was cultured as morphologically stable vesicular structures in a defined, hormone-supplemented PFMR-4 medium. The structures consisted of a single layer of polarized epithelial cells with the apical surface facing the external culture medium, and the basal surface resting on a well-defined basal lamina adjacent to the internal lumen. Vesicles were shown to (a) retain their viability for up to 3 mo. in culture, (b) to actively synthesize DNA after being cultured for over a month in a defined medium, and (c) to respond to steroid hormones. When embedded within a collagen gel, the vesicles reversed their epithelial polarity and formed branching, pseudoglandular structures. It was concluded that the three-dimensional shape of the epithelial vesicles had a critical role to play in their morphological stability nutient requirement, and hormone sensitivity.
Similar content being viewed by others
References
Agnew, W. F.; Alvarez, R. B.; Yuen, T. G. H., et al. A serumfree culture system for studying solute exchanges in the choroid plexus. In Vitro 20:712–722; 1984.
Bettger, W. J.; Boyce, S. T.; Walthall B.J. et al. Rapid clonal growth and serial passage of human diploid fibroblasts in a lipid-enriched synthetic medium supplemented with epidermal growth factor, insulin and dexamethasone. Proc. Natl. Acad. Sci. USA 78:5588–5592; 1981.
Centola, G. M.; Cisar, M.; Knab, D. R. Establishment and morphologic characterization of normal human endometrium in vitro. In Vitro 20:451–462; 1984.
Chen, L.; Lindner, H. R. Mitogenic action of oestradiol 17-β on human myometrial and endometrial cells in long-term tissue cultures. J. Endocrinol. 59:87–97; 1973.
Cline, P. R.; Zamora, P. O.; Hosick, H. L. Morphology and lactose synthesis in tissue culture of mammary alveoli isolated from lactating mice. In Vitro 18:694–702; 1982.
Dohrmann, G. J.; Herdson, P. B. The choroid plexus of the mouse. A microscopic and fine structure study. Zeit. fur Mikr. Anat. Forsch. 82:502–522; 1970.
Dorman, B. H.; Varma, V. A.; Siegfried, J. M., et al.. Morphology and growth potential of stromal cell cultures derived from human endometrium. In Vitro 18:919–928; 1980.
Dumont, J. E. The action of thyrotropin on thyroid metabolism. In: Harris, R. S.; Munson, P. L.; Diczfalusy, E., et al. eds. Vitamins and hormones, vol. 29. London: Academic Press; 1971:287–412.
Garbi, C.; Wollman, S. H. Ultrastructure and some other properties of inverted thyroid follicles in suspension culture. Exp. Cell Res. 138:343–353; 1982.
Hiratsu, T. In vitro cultivation of human endometrium and the influence of steroid hormones on a cell line derived from the endometrium. Kobe J. Med. Sci. 14:29–48; 1968.
Lechner, J. F.; Babcock, M. S.; Marnell, M., et al. Normal human prostate epithelial cell cultures. In: Harris, C. C.; Trump, P. F.; Stoner, G. D., eds. Methods in cell biology vol. 21B. New York: Academic Press; 1980:195–225.
Kirk, D.; King, R. J. B.; Heyes, J., et al. Normal human endometrium in cell culture. I. Separation and characterization of epithelial and stromal components in vitro. In Vitro 14:651–662; 1978.
Lossitzky, S.; Fayet, G; Giraud, A., et al. Thyrotrophin-induced aggregation and reorganization into follicles of isolated porcinethyroid cells. I. Mechanism of action of thyrotrophin and metabolic properties. Eur. J. Biochem. 24:88–99; 1971.
Liszcak, T. M.; Richardson, G. S.; MacLaughlin, D. T., et al. Ultrastructure of human endometrial epithelium in monolayer culture with and without steroid hormones. In Vitro 13:344–356; 1977.
Lynn, D. E.; Oberlander, H.; Ferkovich, S. M. Induction of vesicle formation in a cell line derived from imaginal discs. In Vitro 21:277–281; 1985.
Michaelopoulos, G.; Pitot, H. C. Primary cultures of parenchymal liver cells on collagen membranes. Exp. Cell Res. 94:70–78; 1975.
Orly, J. Growth of functional primary and established rat ovary cell cultures in serum-free medium. In: Barnes, D. W.; Sirbasku, D. A.; Sato, G. H., eds. Cell culture methods for molecular and cell biology, vol. 2. New York: Alan R. Liss, Inc.; 1984:63–87.
Satyaswaroop, P. G.; Bressler, R. S.; de la Pena, M. M., et al. Isolation and culture of human endometrial glands. J. Clin. Endocrinol. Metab. 48:639–641; 1979.
Shannon, J. M.; Pitelka, D. R. The influence of cell shape on the induction of functional differentiation in mouse mammary cells in vitro. In Vitro 17:1016–1028; 1981.
Suard, Y. M. L.; Racine, L.; Kraehenbuhl, J. P. Importance of the topographical organization of rabbit mammary cells in primary cultures for milk protein gene expression and cell proliferation. J. Cell Biol. 91: 229a; 1981.
Tonelli, Q. J.; Sorof, S. Induction of nomal and abnormal differentiation-specific proteins in three-dimensional mammary epithelial cell cultures. J. Cell Biol. 91:26a; 1981.
Trent, J. M.; Davis, J. R.; Payne C. M. The establishment and morphologic characterization of finite cell line from normal human endometrium. Am. J. Obstet. Gynecol. 136:352–362; 1980.
Varma, V. A.; Melin, S. A.; Adamec, T. A., et al. Monolayer culture of human endometrium: Methods of culture and identification of cell type. In Vitro 18:911–918; 1982.
Yang, J. Nandi, S. Growth of cultured cells using collagen as substrate. Int. Rev. Cytol. 81:249–286; 1983.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kirk, D., Alvarez, R.B. Morphologically stable epithelial vesicles cultured from normal human endometrium in defined media. In Vitro Cell Dev Biol 22, 604–614 (1986). https://doi.org/10.1007/BF02623520
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02623520