Summary
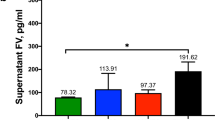
Human diploid fibroblasts were cultured on microcarriers made from DEAE-dextran, denatured collagen, DEAE-dextran linked to denatured collagen, and glass. Cells grown on these four substrates were examined for the production of proteolytic enzymes and arachidonic acid metabolites. Culture fluids from cells grown on the DEAE-dextran microcarriers contained the highest amounts of proteolytic enzyme activity. Both plasminogen-independent and plasminogen-dependent fibrinolytic activities were present and the plasminogen-dependent activity seemed to result from the presence of both urokinase and tissue plasminogen activator. Culture fluid from the cells grown on the glass microcarriers contained the least amount of protease activity, and nearly all of the plasminogen-activator activity seemed to be of the urokinase type. Protease activity in the culture fluids of cells grown on the other two substrates were intermediate. With regard to arachidonic acid metabolites, cells grown on the DEAE-dextran microcarriers produced the highest amounts of cyclooxygenase products but very low levels of lipoxygenase metabolites. Cells grown on the other three substrates produced comparable amounts of various cyclooxygenase products (lower than that produced by cells on the DEAE-dextrans substrate). Cells grown on the glass microcarriers also produced detectable amounts of two lipoxygenase metabolites—leukotriene B4 and leukotriene C4. Inasmuch as both proteolytic enzymes and arachidonic acid metabolites regulate basic cell properties, the differential amount of these metabolites observed in the culture fluids on the various substrates may contribute to the biological differences that exist on these substrates.
Similar content being viewed by others
References
Burger, M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature 227:170–171; 1970.
Chen, L. B.; Buchanan, J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc. Natl. Acad. Sci. USA 72:131–135; 1976.
Coleman, P. L.; Green, G. D. J. A sensitive, coupled assay for plasminogen activator using a thiol ester substrate for plasmin. Ann. NY Acad. Sci. 370:617–626; 1981.
Deutsch, D. G.; Mertz, E. T. Plasminogen: purification from human plasma by affinity chromatography. Science 170:1095–1096; 1970.
Deutsch, H. F. Preparation of immunoglobulin concentrates. In: Methods in immunology and immunochemistry, vol. 1. New York: Academic Press; 1967:315–322.
Fantone, J. C.; Elgas, L. J.; Weinberger, L., et al. Modulation of tumor cell adherence by prostaglandins. Oncology 40:421–426; 1983.
Fantone, J. C.; Marasco, W. A.; Elgas, L. J., et al. Antiinflammatory effects of prostaglandin E1:In vivo modulation of formyl peptide chemotactic receptors on the rat neutrophil. J. Immunol. 130:1495–1497; 1983.
Fenters, J. D.; Fordyce, P. A.; Gerin, J. L., et al. Propagation of Rhinovirus on WI-38 cell monolayers in rolling bottles. App. Microbiol. 15:1460–1466; 1967.
Fowler, R. J.. Drugs which inhibit prostaglandin biosynthesis. Pharmacol. Rev. 26:33–67; 1974.
Gebb, C.; Clark, J. M.; Hirtenstein, M. D., et al. Alternative surfaces for microcarrier culture of animal cells. Dev. Biol. Stand. 50:93–102; 1982.
Giard, D. J.; Thilly, W. G.; Wang, D. I. C., et al. Virus production with a newly developed microcarrier system. App. Environ. Microbiol. 34:668–672; 1977.
Goldberg, A. R.. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell 2:95–102; 1974.
Goldberg, A. R. Involvement of cell and serum proteases in exposing tumor-specific agglutinin sites. Ann. NY Acad. Sci. 234:348–354; 1974.
Goldfarb, R. H. Plasminogen activators. Ann. Rev. Med. Chem. 18:257–264; 1983.
Goldman, D. W.; Goetzl, E. J. Mediation and modulation of immediate hypersensitivity and inflammation by products of the oxygenation of arachidonic acid. In: Ward, P. A., ed. Immunology of inflammation: handbook of inflammation. Elsevier. Amsterdam 4:163–187; 1983.
Goodwin, J. S.; Bankhurst, A. D.; Messner, R. P. Suppression of human T-cell mitogenesis by prostaglandin. J. Exp. Med. 146:1719–1724; 1977.
Goodwin, J. S.; Webb, D. R. Regulation of the immune response by prostaglandins. Clin. Immunol. Immunopathol. 15:106–113; 1980.
Granelli-Piperno, A.; Reich, E. A study of proteases and protease inhibitor complexes in biological fluids. J. Exp. Med. 148:223–234; 1978.
Griffith, J. B.; Thornton, B.; McEntee, I. The development and use of microcarrier and glass sphere culture techniques for the production of herpes simplex viruses. Dev. Biol. Stand. 50:103–110; 1982.
Hatcher, V. B.; Oberman, M. S.; Wertheim, M. S., et al. The relationship between surface protease activity and the rate of cell proliferation in normal and transformed cells. Biochem. Biophys. Res. Commun. 76:602–608; 1977.
Hatcher, V. B.; Wertheim, M. S.; Rhee, C. Y., et al. Relationship between cell surface protease activity and doubling time in various normal and transformed cells. Biochim. Biophys. Acta. 451:499–510; 1976.
Hood, E. G.; Wilson, E. L.; Dowdle, E. B. The regulation of tissue plasminogen activator activity by human fibroblasts. Cell 34:272–279; 1983.
House, W.; Shearer, M.; Maroudas, N. G. Method for bulk culture of animal cells on plastic film. Exp. Cell Res. 71:293–296; 1972.
Hynes, R. O. Role of surface alterations in cell transformation: the importance of proteases and serum proteins. Cell 1:147–156; 1974.
Jimenez de Asua, L.; Clingan, L. D.; Rudland, P. S. Initiation of cell proliferation in cultured mouse fibroblasts by prostaglandin F2α. Proc. Natl. Acad. Sci. USA 72:2724–2728; 1975.
Keese, C.; Giaever, I. Cell growth on liquid microcarriers. Science 219:1448–1449; 1983.
Kunkel, S. L.; Kaercher, K.; Plewa, M., et al. Production of cyclooxygenase products and superoxide anion by macrophages in response to chemotactic factors. Prostaglandins 24:789–799; 1982.
Kunkel, S. L.; Thrall, R. S.; Kunkel, R. G., et al. Suppression of immune complex vasculitis in rats by prostaglandins. J. Clin. Invest. 64:1525–1529; 1979.
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685; 1970.
Laug, W. E.; Jones, P. A.; Benedict, W. F. Relationship between fibrinolysis of cultured cells and malignancy. JNCI 54:173–179; 1975.
Lee, E. C.; Situ, R.; Fantone, J. C., et al. Functional responses of tumor cells to phorbol esters: Role for prostaglandins. Oncology 41:210–216; 1984.
Liebhaber, H.; Pajot, T.; Riordan, J. T. Growth of high titered Rubella virus in roller bottle cultures of Vero cells. Proc. Soc. Exp. Biol. Med. 130:12–14; 1969.
Lowry, O. H.; Rosenbrough, N. J.; Farr, A. L., et al. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275; 1951.
Mahan, M.; Meunier, J.; Newby, M., et al. Prostaglandin E2 production by EL-4 leukemia cells from C57 BL/6 mice: Mechanism for tumor dissemination. JNCI 74:191–195; 1985.
Mensing, H.; Czarnetski, B. M. Leukotriene B4 inducesin vitro fibroblast chemotaxis. J. Invest. Dermatol. 82:9–12; 1984.
Mokashi, S.; Delikatny, E. J.; Orr, F. W. Relationship between chemotaxis, chemokinesis, chemotactic modulators and cyclic nucleotide levels in tumor cells Cancer Res. 43:1980–1983; 1983.
Morley, J.; Bray, M. A.; Jones, R. W., et al. Prostaglandin and thromboxane production by human and guinea-pig macrophages and leukocytes. Prostaglandins 17:729–736; 1979.
Nielsen, V.; Johansson, A. Biosilon, optimal culture conditions and various research scale culture techniques. Dev. Biol. Stand. 46:131–136; 1980.
Obrenovitch, A.; Maintier, C.; Sene, C., et al. Microcarrier culture of fibroblastic cells on modified trisacryl beads. Biol. Cell 46:249–256; 1982.
O'Flaherty, J. T.; Kreutzer, D. L.; Ward, P. A. Effect of prostaglandins E1, E2 and F2α on neutrophil aggregation. Prostaglandins 17:201–210; 1979.
Ossowski, L.; Unkeless, J. C.; Tobia, A., et al. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumour viruses. J. Exp. Med. 137:112–125; 1973.
Ossowski, L.; Quigley, J. P.; Kellerman, G. M.. et al. Fibronolysis associated with oncogenic transformation: Requirement of plasminogen for correlated change in cellular morphology, colony formation in agar and cell migration. J. Exp. Med. 138:1056–1064; 1973.
Pearlstein, E.; Hynes, R. O.; Franks, L. M. et al. Surface proteins and fibrinolytic activity of cultured mammalian cells. Cancer Res. 36:1475–1480; 1975.
Rivkin, I.; Rosenblatt, J.; Becker, E. L. The role of cyclic AMP in the chemotactic responsiveness and spontaneous motility of rabbit peritoneal neutrophils. The inhibition of neutrophil movement and elevation of cyclic AMP levels by catacholamines, prostaglandins, theophylline and cholera toxin. J. Immunol. 115:1126–1134; 1975.
Showell, H. J.; Naccache, P. H.; Sha'af, R. I., et al Inhibition of rabbit neutrophil lysozomal enzyme secretion, non-stimulated and chemotactic factor-stimulated locomotion by nordihydroguaiaretic acid. Life Sci. 27:421–426; 1980.
Smolen, J. E.; Weissmann, G. Effects of indomethacin, 5,8,11,14-eicosatetraynoic acid andp-bromophenacyl bromide on lysozomal enzyme release and superoxide anion generation by human polymorphonuclear leukocytes. Biochem. Pharmacol. 29:533–538; 1980.
Spier, R. E.; Whiteside, J. P. The production of foot-and-mouth disease virus from BHK 21 C 13 cells grown on the surface of DEAE-Sephadex A50 beads. Biotechnol. Bioeng. 18:659–667; 1967.
Snyderman, R.; Goetzl, E. J. Molecular and cellular mechanisms of leukocyte chemotaxis. Science 213:830–836; 1981.
Unkeless, J. C.; Tobia, A.; Ossowski, L., et al. An enzymic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumour viruses. J. Exp. Med. 137:85–111; 1973.
Vanderhoek, J. Y.; Bailey, J. M. Activation of a 15-lipoxygenase/leukotriene pathway in human polymorphonuclear leukocytes by the antiinflammatory agent ibuprofen. J. Biol. Chem. 259:6572–6756; 1984.
Van Wezel, A. L. Growth of cell strains and primary cells on microcarriers. Nature 216:65–66; 1967.
Varani, J.; Dame, M.; Beals, T. F., et al. Growth of three established cell lines on glass microcarriers. Biotechnol. Bioeng. 25:1359–1372; 1983.
Varani, J.; Dame, M.; Rediske, J., et al. Substrate-dependent differences in growth and biological properties of fibroblasts and epithelial cells grown in microcarier culture. J. Biol. Stand. 13:67–76; 1985.
Varani, J.; Orr, W.; Ward, P. A. Comparison of cell attachment and caseinolytic activities of five tumour cell types. J. Cell. Sci. 34:133–134; 1978.
Varani, J.; Orr, W.; Ward, P. A. Cell-associated proteases affects tumor cell migrationin vitro. J. Cell Sci. 36:241–252; 1979.
Varani, J.; Perone, P. Response of Walker 256 carcinosarcoma cells to 12-0-tetradecanoyl phorbol-13-acetate: Possible regulation by endogenous cyclooxygenase metabolites. JNCI 74:165–172; 1985.
Author information
Authors and Affiliations
Additional information
This study was supported in part by grants R44 CA 36656 and IK08HL01332-01 from the Public Health Service, U. S. Department of Health and Human Services and by grant BC-512 from the American Cancer Society. JDH is a research fellow of the American Lung Association.
Rights and permissions
About this article
Cite this article
Varani, J., Hasday, J.D., Sitrin, R.G. et al. Proteolytic enzymes and arachidonic acid metabolites produced by MRC-5 cells on various microcarrier substrates. In Vitro Cell Dev Biol 22, 575–582 (1986). https://doi.org/10.1007/BF02623516
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02623516