Summary
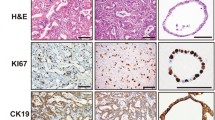
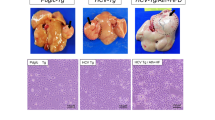
Two Fischer 344 rat hepatoma cell strains, JM1 and JM2, have been isolated from a primary hepatocellular carcinoma. Primary tumor formation was induced in a two-thirds partially hepatectomized rat by a single low dose (70 mg/kg of diethylnitrosamine followed by chronic phenobarbital administration (0.1 g/100 ml drinking water). The primary tumors were passed three times by subcutaneous implantation of tumor fragments into the inguinal region of syngeneic recipients. The fourth pass way by injection of tumor cells directly into the livers of recipient rats. Several weeks later, the tumor-containing rat livers were subjected to collagenase perfusion. Two cell lines emerged from tissue culture of the cells isolated by perfusion. Each cell line was cloned by serial dilution. Cells JM1 and JM2 were tumorigenic when injected into syngeneic rats. The tumors, which arose from injected cell strains, exhibited several characteristics of hepatocellular carcinoma. Morphology was examined by light and electron microscopy. Histochemical studies of JM1 and JM2 cells grown in vitro and in vivo were done. The levels of tyrosine aminotransferase and three microsomal enzymes of importance to drug and carcinogen metabolism were investigated. To our knowledge, this is the first report of cell strains derived from an initiation promotion protocol in rats.
Similar content being viewed by others
References
Morris, H. P.; Slaughter, L. J. Development, growth rate, degree of malignancy, and chromosome pattern of Morris transplantable hepatomas. Lapis, K.; Johannessen, J. V., eds. Liver carcinogenesis. New York, NY: Hemisphere Publishing Corp.; 1979: 263–282.
Farber, E. The sequential analysis of liver cancer induction. Biochim. Biophys. Acta 605: 149–166; 1980.
Craddock, V. M.. Liver cell cancer. Cameron, H. M.; Linsell, D. S.; Warwick, G. P. eds. Cell proliferation and experimental liver cell cancer. Amsterdam: Elsevier; 1976: 153–201.
Cameron, R.; Sweeny, G. D.; Jones, K.; Lee, G.; Farber, E. A relative deficiency of cytochrome P-450 and aromatic hydrocarbon (Benzo(alpha)pyrene) hydroxylase in hyperplastic nodules induced by acetylaminofluorene in rat liver. Cancer Res. 36: 3888–3893; 1976.
Peraino, C.; Fry, R. J. M.; Staffeldt, E.; Kisielski, W. E. Effect of varying the exposure to phenobarbital on its enhancement of 2-acetylamino-fluorene-induced hepatic tumorigenesis. Cancer Res. 33: 2701–2705; 1973.
Pitot, H. C.; Barsness, L.; Goldsworthy, T.; Kitagawa, T. Biochemical characterization of stages of hepatocarcinogenesis after a single dose of diethylnitrosamine. Nature 271: 456–458; 1978.
Deschenes, J.; Valet, J. P.; Marceau, N. Hepatocytes from newborn and waenling rats in monolayer culture: Isolation by perfusion, fibronectin-mediated adhesion, spreading and functional activities. In Vitro 16: 722–730; 1980.
Seglen, P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 13:29–83; 1976.
Elmore, E.; Swift, M. Growth of human skin fibroblasts in dialyzed fetal bovine serum. In Vitro 13: 837–842; 1977.
Rutenberg, A. H.; Kim, H.; Fischbein, J. W.; Hanker, J. S.; Wasserkrug, H. L.; Seligman, A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J. Histochem. Cytochem. 17: 517–527; 1969.
Wachstein, M.; Meisel, E. Histochemistry of hepatic phosphatases at a physiological pH with special reference to the demonstration of bile canaliculi. Am. J. Clin. Pathol. 27: 12–23; 1957.
Michalopoulos, G.; Pitot, H. C. Primary culture of parenchymal liver cells on collagen membranes. Exp. Cell Res. 94: 70–78; 1975.
Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes. J. Biol. Chem. 239: 2370–2378; 1964.
Phillips, A. H.; Langdon, R. G. Hepatic triphosphopyridine nucleotide cytochrome P-450 reductase. Isolation, characterization and kinetic studies. J. Biol. Chem. 237: 2652–2660; 1962.
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 72: 248–254; 1976.
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685; 1970.
Granner, D. K.; Tomkins, G. M. Tyrosine aminotransferase (rat liver). Methods Enzymology. 17(A): 633–637; 1970.
Talanko, H.; Ruoslanti, E. Differential expression of alpha-fetoprotein and gamma-glutamyl transpeptidase in chemical and spontaneous hepatocarcinogenesis. Cancer Res. 39: 3495–3501; 1979.
Cameron, R.; Keller, A.; Kolin, A.; Malkin, A.; Farber, E. Gamma-glutamyl transferase in putative premalignant liver cell populations during hepatocarcinogenesis. Cancer Res. 38: 823–829; 1978.
Jacobsen, J. E.; Putnam, J. E.; Matyas, G. R.; Morre, D. J. Gammaglutamyl transpeptidase activity of transformed rat hepatocytes. In Vitro 18: 285; 1982.
Miyake, Y.; Gaylor, J. L.; Morris, H. P. Abnormal microsomal cytochromes and electron transport in Morris hepatomas. J. Biol. Chem. 249(6): 1980–1987; 1974.
Ryan, D. E.; Thomas, P. E.; Korzeniowski, D.; Levin, W. Separation and characterization of highly purified forms of liver microsomal cytochrome P-450 from rats treated with polychlorinated biphenyls, phenobarbital and 3-methylcholanthrene. J. Biol. Chem. 254(4): 1365–1374; 1979.
Harada, N.; Omura, T. Selective induction of two different molecular species of cytochrome P-450 by phenobarbital and 3-methylcholanthrene. J. Biochem. 89: 237–248; 1981.
Saito, T.; Strobel, H. W. Purification to homogeneity and characterization of a form of cytochrome P-450 with high specificity for benzo(a)pyrene from beta-naphthoflavone-pretreated rats. J. Biol. Chem. 256: 984–988; 1981.
Van Rijn, H.; Bevers, M. M.; van Wijk, R.; Wicks, W. D. Regulation of phosphoenolpyruvate carboxykinase and tyrosine transaminase in hepatoma cell cultures III. Comparative studies in H35, HTC, MH1C1, and RLC cells. J. Cell Biol. 60: 181–191; 1974.
Diamondstone, T. I. Assay of tyrosine transaminase activity by conversion ofp-hydroxyphenylpyruvate top-hydroxybenzaldehyde. Anal. Biochem. 16: 395–401; 1966.
Judah, D. J.; Legg, R. F.; Neal, G. E. Development of resistance to cytotoxicity during aflatoxin carcinogenesis. Nature 265: 343–345; 1977.
Conney, A. H.; Brown, R. R.; Miller, J. A.; Miller, E. C. The metabolism of methylated aminoazo dyes VI. Intracellular distribution and properties of the demethylase system. Cancer Res. 17: 628–633; 1957.
Adamson, R. H.; Fouts, J. R. The metabolism of drugs by hepatic tumors. Cancer Res. 21: 667–672; 1957.
Potter, V. R. Transplantable animal cancer, the primary standard (guest editorial). Cancer Res. 21: 1331–1333; 1961.
Conney, A. H.; Burns, J. J. Induced synthesis of oxidative enzymes in liver microsomes. Adv. Enzyme Regul. 1: 189–214; 1963.
Hart, L. G.; Adamson, R. H.; Morris, H. P.; Fouts, J. R. The stimulation of drug metabolism in various rat hepatomas. J. Pharmacol. Exp. Ther. 149: 7–15; 1965.
Nebert, D. W.; Mason, H. S. A microsomal difference between normal liver and “minimal-deviation” hepatoma 5123 detectable by electron spin resonance. Biochim. Biophys. Acta 86: 415–417; 1964.
Sugimura, T.; Ikeda, K.; Hirota, K.; Hozumi, M.; Morris, H. P. Chemical and enzymatic and cytochrome assays of microsomal fraction of hepatomas with different growth rates. Cancer Res. 26: 1711–1716; 1966.
Coon, M. J.; Haugen, D. A. Induction of multiple forms of mouse liver cytochrome P-450. J. Biol. Chem. 251: 1817–1827; 1976.
Kohli, K. K.; Linko, P.; Goldstein, J. A. Multiple forms of solubilized and partially resolved cytochrome P-450 from rats induced by 2,3,5,2′,3′,5′ and 3,4,5,3′,4′,5′ hexachlorobiphenyls. Biochem. Biophys. Res. Commun. 100: 483–490; 1981.
Welton, A. F.; O'Neal, F. O.; Chaney, L. C.; Aust, S. D. Multiplicity of cytochrome P-450 hemoproteins in rat liver microsomes. J. Biol. Chem. 250: 5631–5639; 1975.
Emerman, J. T.; Pitelka, D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro 13: 316–328; 1977.
Chambard, M.; Gabrion, J.; Mauchamp, J. Reorganization in culture of thyroid follicular cells-influence of collagen on the orientation of cell polarity. C. R. Acad. Sci. (Paris) 291: 79–81; 1980.
Michalopoulos, G.; Sattler, G. L.; Pitot, H. C. Maintenance of microsomal cytochromes b5 and P-450 in primary cultures of parenchymal liver cells. Life Sci. 18: 1139–1144; 1976.
Strom, S. C.; Michalopoulos, G. Collagen as a substrate for cell growth and differentiation. Meth. Enzymol. 82: 544–555; 1982.
Yamada, K. M.; Yamada, S. S.; Pastan, I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc. Natl. Acad. Sci. USA 73: 1217–1221; 1976.
Author information
Authors and Affiliations
Additional information
These studies were supported by Grants CA-30241 and CA-26904 from the National Institutes of Health, Bethesda, MD, and Collaborative Research Agreement 808549 from the Environmental Protection Agency.
Rights and permissions
About this article
Cite this article
Novicki, D.L., Jirtle, R.L. & Michalopoulos, G. Establishment of two rat hepatoma cell strains produced by a carcinogen initiation, phenobarbital promotion protocol. In Vitro 19, 191–202 (1983). https://doi.org/10.1007/BF02618059
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02618059