Abstract
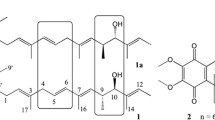
The inhibitory effects on poly(U)-directed polyphenylalanine synthesis of cryptopleurine and a number of structurally related analogs have been compared in a yeast cell-free system. Results suggest that the quinolidine structure by itself does not promote biological activity, and for an inhibitory effect it must be condensed with a phenanthrene or another related compound such as naphthalene. The results are presented and possible relationships between structure and activity for the compounds emetine, tubolosine, tylophora alkaloids, and various cryptopleurine analogs are considered.
Similar content being viewed by others
Literature Cited
González Trigo, G., Alvarez-Builla, J., Söllhuber, M. 1978. N ⊂[2-(4-metoxifenil)-3-(3,4-dimetoxifenil)]alil⊃ piperidina. Sintesis de un análogo de criptopleurina. Anales de Quimica de la Real Sociedad Española de Física y Quimica74: 523–526.
Grant, P., Sanchez, L., Jiménez, A. 1974. Cryptopleurine resistance genetic locus for a 40S ribosomal component inSaccharomyces cerevisiae. Journal of Bacteriology120: 1308–1314.
Gupta, R. S., Siminovitch, L. 1977. The molecular basis of emetine resistance in Chinese hamster ovary cells: Alteration in the 40S ribosomal subunit. Cell10: 61–66.
Gupta, R. S., Siminovitch, L. 1977. Mutants of CHO cells resistant to the protein synthesis inhibitors, cryptopleurine and tylocrebrine: Genetic and biochemical evidence for common site of action of emetine, cryptopleurine, tylocrebrine and tubulosine. Biochemistry16: 3209–3214.
Sánchez, L., Vázques, D., Jiménez, A. 1977. Genetics and biochemistry of cryptopleurine resistance in the yeastSaccharomyces cerevisiae. Molecular and General Genetics156: 319–326.
Trigo, G. G., Gálvez, E., Söllhuber, M. 1980. Synthesis and conformational analysis of 7,8-diphenyl-1,3,4,6,9,9a-hexahydro-2H-quinolizines, Journal of Heterocyclic Chemistry, in press.
Vázquez, D. 1979. Inhibitors of protein biosynthesis. Berlin-Heidelberg-New York: Springer-Verlag.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Söllhuber, M., Grande, M.T., Trigo, G.G. et al. Structure-activity relationships between cryptopleurine and related compounds acting on yeast cell-free systems. Current Microbiology 4, 81–84 (1980). https://doi.org/10.1007/BF02602897
Issue Date:
DOI: https://doi.org/10.1007/BF02602897