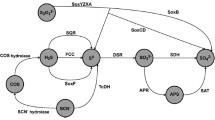
Abstract
A thiosulfate-oxidizing enzyme was partially purified fromChromatium vinosum, and some of its properties were studied. The enzyme rapidly reducede HiPIP (high-potential nonheme iron protein) in the presence of thiosulfate. Cytochromesc of yeast and tuna and ferricyanide also acted well as electron acceptors for the enzyme; horse cytochromec was a poor electron acceptor. Cytochromec-552, cytochromec′, and cytochromec-553 did not act as electron acceptors. The enzyme was inhibited by cyanide and sulfite. On the basis of the stoichiometry in reduction of ferricyanide catalyzed by the enzyme in the presence of thiosulfate, the oxidized product of thiosulfate was inferred to be tetrathionate.
Similar content being viewed by others
Literature Cited
Bartsch, R. G. 1971. High potential iron proteins: Bacterial, pp. 644–649. In: San Pietro A. (ed.), Methods in enzymology, vol. 23A. New York: Academic Press.
Bartsch, R. G. 1978. Purification of (4Fe-4S)1−2−ferredoxins (high-potential iron-sulfur proteins) from bacteria, pp. 329–340. In: Fleischer, S., Packer, L. (eds.), Methods in enzymology, vol. III. New York: Academic Press.
Bartsch, R. G., Kamen, M. D. 1960. Isolation and properties of two soluble heme proteins in extract of the photoanaerobeChromatium. Journal of Biological Chemistry235:825–831.
Carter, C. W., Jr., Kraut, J., Freer, S. T., Xuong, N.-H., Alden, R. A., Bartsch, R. G. 1974. Two-angstrom crystal structure of oxidizedChromatium high potential iron protein. Journal of Biological Chemistry249:4212–4225.
Connelly, J. L., Morrison, M., Stotz, E. 1958. Hemins of beef heart muscle. Journal of Biological Chemistry233:743–747.
Cusanovich, M. A., Bartsch, R. G. 1969. A high potential cytochromec fromChromatium chromatophores. Biochimica et Biophysica Acta189:245–255.
Dus, K., Tedro, S., Bartsch, R. G. 1973. The complete amino acid sequence ofChromatium high potential iron sulfur protein. Journal of Biological Chemistry248:7318–7331.
Evans, M. C. W., Lord, A. V., Reeves, S. G. 1974. The detection and characterization by electron-paramagnetic-resonance spectroscopy of iron-sulphur proteins and other electron-transport components in chromatophores from the purple bacteriumChromatium. Biochemical Journal138:177–183.
Fukumori, Y., Yamanaka, T. 1979. Flavocytochromec ofChromatium vinosum. Some enzymatic properties and subunit structure. Journal of Biochemistry85:1405–1415.
Hagihara, B., Tagawa, K., Nozaki, M., Morikawa, I., Yamashita, J., Okunuki, K. 1957. Crystallization of cytochromec from fish. Nature179:249–251.
Hashwa, F., Pfennig, N. 1972. The reductive enzymatic cleavage of thiosulfate. Methods and application. Archives of Microbiology81:36–44.
Kusai, A., Yamanaka, T. 1973. The oxidation mechanisms of thiosulphate and sulphide inChlorobium thiosulfatophilum. Roles of cytochromec-551 and cytochromec-553. Biochimica et Biophysica Acta325:304–314.
Peck, D. H., Jr., Deacon, T. E., Davidson, J. T. 1965. Studies on adenosine t′-phosphasulfate reductase fromDesulfovibrio desulfuricans andThiobacillus thioparus. I. The assay and purification. Biochimica et Biophysica Acta96:429–446.
Smith, A. J. 1966. The role of tetrathionate in the oxidation of thiosulphate byChromatium sp. strain D. Journal of General Microbiology42:371–380.
Smith, A. J., Lascelles, J. 1966. Thiosulphate metabolism and rhodanese inChromatium sp. Journal of General Microbiology42:357–370.
van Niel, C. B. 1963. A brief survey of the photosynthetic bacteria, pp. 459–467. In: Gest, H., San Pietro, A., Vernon, L. P. (eds.), Bacterial photosynthesis. Yellow Springs, Ohio: Antioch Press.
Yamanaka, T., Fukumori, Y. 1979. A biochemical comparison betweenChlorobium andChromatium flavocytochromesc. In: Yagi, K., Yamano, T. (eds.) Proceedings of the Sixth International Symposium on Flavins and Flavoproteins. Tokyo: Japan Scientific Societies Press. In press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fukumori, Y., Yamanaka, T. A high-potential nonheme iron protein (HiPIP)-linked, thiosulfate-oxidizing enzyme derived fromChromatium vinosum . Current Microbiology 3, 117–120 (1979). https://doi.org/10.1007/BF02602443
Issue Date:
DOI: https://doi.org/10.1007/BF02602443