Abstract
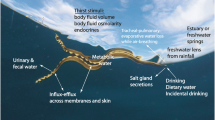
In vivo diffusion-weighted magnetic resonance imaging (MRI) was used to determine the effects of an osmotic challenge (1% NaCl) to a freshwater fish, the common carp (Cyprinus carpio). The imaged region covered organs such as the swimbladder, the liver, the kidney, the intestine, the spinal cord, and muscle tissue. A striking difference between salt-treated and control fish was found in the liver. The apparent diffusion coefficient value of livers from control fish was (0.39±0.16) 10−9 m2/s and of salt-treated fish was (1.23±0.14) 10−9 m2/s, which points to an increase in extracellular water content. These results were partially confirmed by a decrease in dry/wet weight ratio of the liver tissue. We also found increased levels of stress proteins in liver tissue. TheQ factor of the applied radiofrequency coil dropped dramatically when we performed experiments with salt-exposed fish, indicating an increased conductivity resulting from the increased ion concentration and osmolarity of the fish. The data on plasma osmolarity of salt-exposed fish confirm a significant osmolarity increase upon salt exposure (from 334 to 430 mOsm/kg) and exceeded the osmolarity of the salt water (324 mOsm/kg), indicating that carp tend to cope with an increased salinity by increasing the internal osmolarity (hyperosmotic regulation). These data demonstrate that diffusion-weighted MRI might be a useful and noninvasive tool in the study of osmotic challenges of aquatic organisms.
Similar content being viewed by others
References
Stark DD, Bradley WG (1988)Magnetic Resonance Imaging, 1st ed. St. Louis, C.V. Mosby.
Blackband SJ, Stotskopf MK (1990)In vivo nuclear magnetic resonance imaging and spectroscopy of aquatic organisms.Magn Reson Imaging 8: 191–198.
Brouwer M, Engel DW, Bonaventura J, Johnson GA (1992)In vivo magnetic resonance imaging of the blue crab,Callinectes sapidus: effect of cadmium accumulation in tissues on proton relaxation properties.J Exp Zool 263: 32–40.
Gassner G, Vogelbein W, Line M (1992) Magnetic resonance detection of environmentally induced hepatic lesions inFundulus heteroclitus.Mar Environ Res 34: 7–11.
Rand GM (1995)Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment, 2nd ed. Washington D.C.: Taylor & Francis.
Heath AG (1995)Water Pollution and Fish Physiology, 2nd ed., Boca Raton: CRC Press.
Sanders BM (1993) Stress proteins in aquatic organisms: an environmental perspective.Crit Rev Toxicol 23: 49–75.
Versteeg DJ, Giesy JP (1986) The histological and biochemical effects of cadmium exposure in the bluegill sunfish (Lepomis macrochirus).Ecotoxicol Environ Safety 11: 31–43.
Lowe DM, Pipe RK (1994) Contaminant induced lysosomal membrane damage in marine mussel digestive cells: anin vitro study.Aquat Toxicol 30: 357–365.
Evans DH (1993)The Physiology of Fishes, 2nd ed. Boca Raton: CRC Press.
Kirsch R, Lahlou B (1987)Comparative Physiology of Environmental Adaptations. I. Adaptations to Salinity and Dehydration. Basel: Karger.
Gupta OP, Hanke W (1982) The effects of osmotic stressors on the stenohaline carp (Cyprinus carpio).Comp Biochem Physiol 71A 165–173.
Kulijev ZM, Agayarova AE (1984) Ecological-morphometrical characteristics of wild carp,Cyprinus carpio (Cyprinidae), of the central and southern Caspian.J. Ichthyol 24: 9–17.
Schildhauer B, Debus L, Schildhauer V, Heise R, Mieske B (1992) Large-scale trial at the southern Baltic coast on the extensive management of mirror carp (Cyprinus carpio L.) in a hypertrophic brackish water.Progress in Aquaculture Research: Proceedings of the Fourth German-Israeli Status Seminar, pp 137–152.
Lam TJ, Sharma R (1985) Effects of salinity and thyroxin on larval survival, growth and development in the carp,Cyprinus carpio.Aquaculture 44: 201–212.
Schildhauer B (1983) Untersuchungen zur Salzge-haltsverträglichkeit juveniler Karpfen (Cyprinus carpio L.) und Marmorkarpfenhybriden (Aristichthys nobilis RICH. XHypophtalmichthys molitrix VAL.).Fisch-Forsch Wiss Schriften 21: 24–30.
De Boeck G, Nilsson GE, Vlaeminck A, Blust R (1996) Central monoaminergic responses to salinity and temperature rises in common carp.J Exp Biol 199 1605–1611.
Abo Hegab S, Hanke W (1982) Electrolyte changes and volume regulatory processes in the carp (Cyprinus carpio) during osmotic stress.Comp Biochem Physiol 71A: 157–164.
Hossmann KA, Fischer M, Bockhorst K, Hoehn-Berlage M (1994) NMR imaging of the apparent diffusion coefficient (ADC) for the evaluation of metabolic suppression and recovery after prolonged cerebral ischemia.J Cereb Blood Flow Metab 14: 723–731.
van der Toorn A, Syková E, Dijkhuizen RM, Vorisek I, Vargová L, Skobisova E, van Lookeren Campagne M, Reese T, Nicolay K (1996) Dynamic changes in water ADC, energy metabolism, extracellular space volume, and tortuosity in neonatal rat brain during global ischemia.Magn Reson Med 36: 52–60.
van Gelderen P, de Vleeschouwer HM, Despres D, Pekar J, van Zijl PCM, Moonen CTW (1994) Water diffusion and acute stroke.Magn Reson Med 31: 154–163.
Clesceri LS, Greenberg AE, Trussel RR (1989)Standard Methods for the Examination of Water and Waste Water, 17th ed. Baltimore: Port City Press.
Sovio A, Nyholm K, Huhti M (1977) Effects of anaesthesia with MS 222, neutralized MS 222 and benzocaine on the blood constituents of rainbow trout,Salmo gairdneri.J Fish Biol 10: 91–101.
Maloiy GMO (1979)Comparative Physiology of Osmoregulation in Animals. Orlando: Academic Press.
Gonzalez CRM, Bradley BP (1994) Are there salinity stress proteins?Mar Environ Res 39: 205–208.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Boeck, G., Vanaudenhove, M., Verhoye, M. et al. Water household of the common carp,Cyprinus carpio, when submitted to an osmotic challenge, as determined by diffusion-weighted magnetic resonance imaging at 7 T. MAGMA 5, 13–19 (1997). https://doi.org/10.1007/BF02592260
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02592260