Summary
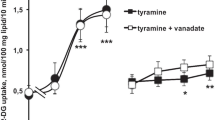
The insulin receptor is not the site for the stimulatory effect of copper on glucose incorporation into total lipids by adipose tissue; prewashing of adipose tissue of rats fed a stock diet in an insulin-free medium increases the glucose incorporation into total lipids in the presence of 0.1 unit insulin from 220 above control to 430% in the nondiabetic and from 154 to 230% in the streptozotocin-diabetic rat. In contrast, glucose incorporation in the presence of CuCl2 · 2H2O is unaffected by prewashing, being the same in the prewashed as in the unwashed adipose tissue. On the other hand, mild trypsin digestion of adipocytes decreases the glucose incorporation in the presence of 28.5 mU insulin, from 201 to 126% — whereas the effect of copper on glucose incorporation is the same in the trypsin-treated or untreated adipocyte. In order to obtain maximal copper effect the adipocyte has to be preconditioned by insulin; preincubation of diabetic adipose tissue first for one hour with 0.1 unit insulin, and another hour with 100 µg CuCl2 · 2H2O added to the medium, results in greater glucose incorporation (230% above control) than when incubated alone with either CuCl2 · 2H2O (125%) or insulin (154%) for two hours. In addition to its previously noted effect on thein vivo insulin release, copper increased the number of the insulin receptor sites in adipocytes.
Similar content being viewed by others
References
Adams K. F., Johnson G., Hornonski K. E., Lineberger T. H.: The effect of copper on erythrocyte deformability. A possible mechanism of hemolysis in acute copper intoxication — Biochim. biophys. Acta (Amst.)550, 279, 1979.
Cohen A. M., Fields M.: The effect of copper on carbohydrate metabolism — Fed. Proc.41, 784, 1982.
Cohen A. M., Teitelbaum A., Miller E., Ben-Tor V., Hirt R., Fields M.: Effect of copper on carbohydrate metabolism in rats — Israel J. med. Sci.18, 840–44, 1982.
Czech M. P.: Differential effects of sulfhydryl reagents on activation and deactivation of the fat cell hexose transport system — J. biol. Chem.251, 1164, 1976.
Czech M. P., Fain J. N.: Insulin protection against fat cell receptor inactivation by trypsin — Endocrinology87, 191, 1970.
Czech M. P., Lawrence J. C. Jr., Lynn W. S.: Evidence of the involvement of sulfhydryl oxidation in the regulation of fat cell hexose transport by insulin — Proc. nat. Acad. Sci. (Wash.)71, 4173, 1974.
Ferreira K. T. G.: The effect of copper on frog skin. The role of sulphydryl groups — Biochim. biophys. Acta (Amst.)511, 298, 1978.
Fields M., Ferretti R. J., Smith J. C., Reiser S.: Effect of copper deficiency on metabolism and mortality in rats fed sucrose or starch diets — J. Nutr.113, 1335, 1983.
Folch J., Lees M., Sloane-Stanley G. H.: A simple method for the isolation and purification of total lipids from animal tissue — J. biol. Chem.226, 497, 1957.
Gavin J. R., Roth J., Neville D. M., De Meyts P. D., Buell D. M.: Insulin dependent regulation of insulin receptor concentration in cell culture — Proc. nat. Acad. Sci. (Wash.)7, 84, 1974.
Hassel C. A., Marchello J. A., Lei K. Y.: Impaired glucose tolerance in copper-deficient rats — J. Nutr.113, 1081, 1983.
Kono T.: Destruction and restoration of the insulin effector system of isolated fat cells — J. biol. Chem.244, 5777, 1969.
Livingston J. M., Amatruda J. M., Lockwood D. H.: Studies of glucose transport system of fat cells: effects of insulin and insulin mimickers — Amer. J. Physiol.235, E 484, 1978.
Loten E. G., LeMarchand Y. L., Jeanenet F. A., Denton R. M., Jeanrenaud B.: Does hyperinsulinemia in ob/ob mice cause an insulin stimulated adipose tissue? — Amer. J. Physiol.230, 602, 1976.
Lowry D. H., Rosenbrough W. J., Farr A. L., Randall R. J.: Protein measurements with the Folin-phenol reagent — J. biol. Chem.193, 265, 1951.
Madar Z., Teitelbaum A., Miller E., Cohen A. M.: Genetic differences in insulin receptors in adipocytes of diabetic rats. In:Shafrir E., Renold A. E. (Eds): Lessons from animal diabetes. John Libbey & Co., Ltd., London-Paris, 1984; p. 110–120.
Rodbell M.: Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis — J. biol. Chem.239, 375, 1964.
Saggerson E. D., Sooranna S. R., Evans C. J.: Insulin-like action of nickel and other transition-metal ions in rat fat cell — Biochem. J.154, 349, 1976.
Winand J., Christophe J.: Effect of preincubation on the metabolism of (U-14C) glucose and (3H) H2O by the epididymal adipose tissue of obese-hyperglycemic (ob/ob) obese-hyperglycemic (ob/ob) Bar Harbor mice — Arch. int. Physiol. Biochim.84, 207, 1976.
Winand J., Dehaye J. P., Christophe J.: Regulation of adipose tissue lipid metabolism in obese-hyperglycemic mice — Biochem. Soc. Trans.5, 903, 1977.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cohen, A.M., Miller, E. & Madar, Z. Is copper effect on glucose incorporation mediated by the insulin receptor in rat adipose tissue?. Acta diabet. lat 22, 47–54 (1985). https://doi.org/10.1007/BF02591092
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02591092