Abstract
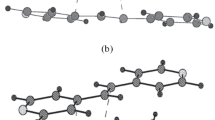
This paper studies the crystal structure of new substituted light-sensitive azomethine N-oxides (nitrones): C-2′-(o-oxyphenyl)vinyl-N-p-methylphenyl nitrone (1), C-2′-(2″-oxy-5″-bromophenyl)vinyl-N-p-methylphenyl nitrone (2), C-2′-(2″-oxy-5″-bromophenyl)-vinyl-N-phenyl nitrone (3), and C-2′-(o-oxyphenyl)vinyl-N-methyl nitrone (4). In contrast to the compounds studied earlier [1, 2], C-2′-(β-oxy-α-naphthyl)vinyl-N-p-methylphenyl nitrone (5), C-2′-(β-oxy-α-naphthyl)vinyl-N-phenyl nitrone (6), C-2′-(o-oxyphenyl) vinyl-N-phenyl nitrone (7), and C-2′-(o-oxyphenyl)vinyl-N-p-bromophenyl nitrone (8), the nitrones studies in this work have anti-rather than syn-orientations of the nitrone and hydroxyl groups. Due to this spatial arrangement of the proton-donating hydroxyl and proton-accepting nitrone groups, molecules in crystals 1–4 are bonded by intermolecular hydrogen bonds (IHB) to form chains but not centrosymmetric dimeric associates (CDA). Two types of chain arrangements were revealed: “head-to-tail” and “head-to-tail, tail-to-head”. It is shown that the introduction of an alkyl substituent instead of an aryl one at the nitrogen atom of the nitrone group in 4 leads to a change in the geometry of the IHB in the H-associate. It is proven that the hydroxyl proton can undergo an intermolecular O→O transfer in the chain of hydrogen bonds in crystals 1–4, which can give rise to photochemical transformations in these crystals.
Similar content being viewed by others
References
S. M. Aldoshin, A. N. Utenyshev, I. I. Chuev, et al.,Mol. Cryst. Liq. Cryst.,220, 231 (1992).
A. N. Utenyshev, S. M. Aldoshin, I. I. Chuev, et al. (in press).
G. M. Sheldrick,SHELX-76. Program for Crystal Structure Determination, Cambridge University (1976).
G. V. Timofeeva, N. J. Chernikova, and P. M. Zorkii,Usp. Khim.,69, 966 (1980).
L. M. Babkov, G. A. Puchkovskaya, S. P. Makarenko, and T. A. Gavrilko,Spectroscopy of Molecular Crystals [in Russian], Naukova Dumka, Kiev (1989).
Additional information
Institute of Chemical Physics in Chernogolovka, Russian Academy of Sciences. Translated fromZhurnal Strukturnoi Khimii, Vol. 37, No. 2, pp. 349–362, March–April, 1996.
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Utenyshev, A.N., Aldoshin, S.M. Formation of intermolecular hydrogen bonds in light-sensitive crystals of C-2′-(o-oxyphenyl)vinyl-N-p-methylphenyl nitrone, C-2′-(2″-oxy-5″-bromophenyl)vinyl-N-p-methylphenyl nitrone, C-2′-(2″-oxy-5″-bromophenyl)-vinyl-N-phenyl nitrone, and C-2′-(o-oxyphenyl)vinyl-n-methyl nitrone. J Struct Chem 37, 305–317 (1996). https://doi.org/10.1007/BF02591061
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02591061