Abstract
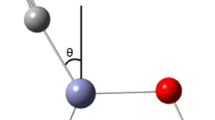
This paper gives the results of quantum chemical MINDO/3 calculations of carbon monoxide adsorption on the ZnO polar (0001) surface. The energetically most favorable one-center adsorption of carbon monoxide on the ZnO (0001) surface occurs by the electron density transfer from the lone electron pair of CO carbon to the vacant orbital of the Zn 2+3C cation. The calculated heat of CO adsorption, dependent on the type of covering, and the stretching frequency υCO are in good agreement with the available experimental data.
Similar content being viewed by others
References
K. Klier,Adv. Catal.,31, 243–313 (1982).
P. Courty, D. Durand, E. Freund, and A. Sugier,J. Mol. Catal.,17, 241–254 (1982).
A. L. Dent and R. J. Kokes,J. Phys. Chem.,73, 3772–3780 (1969).
R. J. Kokes,Acc. Chem. Res.,6, 226–233 (1973).
C. C. Chang and R. J. Kokes,J. Catal.,28, 92–100 (1973).
R. Sekine, H. Adachi, and T. Morimoto,Surf. Sci.,208, 177–188 (1989).
R. Kuwabara, H. Adachi, and T. Morimoto, ibid.,193, 271–286 (1988).
J. A. Tossel,Inorg. Chem.,16, 2944–2951 (1977).
I. Gopel, J. Pollmann, I. Ivanov, and B. Riehl,Phys. Rev. B.,26, 3144–3157 (1982).
A. B. Anderson and J. A. Nichols,J. Am. Chem. Soc.,108, 1385–1388 (1986).
H. Nakatsuji and Y. Fukunishi,Intern. J. Quant. Chem.,42, 1101–1114 (1992).
J. A. Rodriguez and Ch. T. Campbell,Surf. Sci.,194, 475–504 (1988).
N. U. Zhanpeisov and G. M. Zhidomirov,Zh. Struk. Khim.,33, 151–153 (1992).
N. U. Zhanpeisov, A. G. Pelmenshchikov, and G. M. Zhidomirov,Kinet. Katal.,31, 563–569 (1990).
S. C. Abrahams and J. L. Bernstein,Acta Crystallogr. Sec. B.,125, 1233–1247 (1969).
R. R. Gay, M. H. Nodine, V. E. Henrich, et al.,J. Am. Chem. Soc.,102, 6752–6761 (1980).
L. L. D'Amico, F. R. McFeely, and E. I. Solomon,J. Am. Chem. Soc.,105, 6380–6383 (1983).
A. L. Waddams,Chemicals from Petroleum, Wiley, New York (1973).
Additional information
Institute of Catalysis, Siberian Branch, Russian Academy of Sciences. Ruhr University, Bochum, Germany. Translated fromZhurnal Strukturnoi Khimii, Vol. 35, No. 1, pp. 12–16, January–February, 1994.
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Zhanpeisov, N.U., Zhidomirov, G.M. & Baerns, M. Cluster quantum chemical study of the interaction between a carbon monoxide molecule and a zinc oxide surface. J Struct Chem 35, 9–12 (1994). https://doi.org/10.1007/BF02578495
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02578495