Abstract
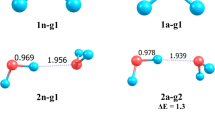
The energies and structures of many small water clusters (H2O)n (n=8-26) are calculated using the atom-atom potential functions suggested earlier. For each n, several stable configurations were found that differ in the number of H-bonds and in the topology of the graphs formed by such bonds. The clusters in which the molecules lie at the vertices of convex polyhedra have the lowest-energy but other configurations may have close or even lower energies. For the most stable clusters, the energy dependence on n is close to linear. At 300 K, the mean energies of the clusters behave similarly. Monte-Carlo simulations showed that the clusters undergo pseudomelting at approximately 200 K.
Similar content being viewed by others
References
M. V. Kirov,Zh. Strukt. Khim.,34, No. 4, 77–82 (1993).
T. Mark and A. W. Castleman, inAdvances in Atomic Molecular Physics D. Bates and B. Bederson (eds.), New York (1985), p. 65.
F. F. Abraham,Homogeneous Nucleation Theory, Academic Press, New York (1974).
A. A. Vigasin,Zh. Strukt. Khim. 24, No. 1, 116–141 (1983).
G. G. Malenkov inWater in Disperse Systems [in Russian], B. V. Deryagin et al. (eds.), Khimiya, Moscow (1989), pp. 132–147.
A. Vigiri and S. C. Farantos,J. Chem. Phys.,98, No. 5, 4059–4075 (1993).
D. J. Wales and I. Ohmini, ——ibid.98 No. 5, 7245–7268 (1993).
J. D. Bernal and R. Fowler, ——ibid.,1, No. 5, 515–548 (1933).
G. Nemethy and H. Scheraga, ——ibid.,36, 3382–3388 (1962).
G. G. Malenkov, inPhysical Chemistry. Current Problems [in Russian], Ya. M. Kolotyrkin (ed.), Khimiya, Moscow (1984), pp. 41–76.
V. I. Poltev, T. A. Grokhlina, and G. G. Malenkov,J. Biomol. Struct Dynam.,2, 413–429 (1984).
W. Saenger,Nature,79, 343–345 (1979).
P. L. M. Plummer and B. N. Hale,J. Chem. Phys.,56, 4329–4334 (1972).
I. P. Buffey, W. B. Brown, and H. A. Gebbie,Chem. Phys. Lett.,148, No. 4, 281–284 (1988).
G. A. Jeffrey and W. Saenger,Hydrogen Bonding in Biological Systems Springer, Berlin (1991).
U. Nakahima, H. Shinohara, N. Noboyuki, and H. Tanaka,J. Chem. Phys.,84, No. 1, 209–214 (1984).
S. Wei, Z. Shi, and A. W. Castleman, ——ibid.,94, No. 1, 3268–3270 (1991).
G. Torchet, P. Schwartz, J. Farges, et al., ——ibid. 79, No. 12, 6196–6203 (1983).
C. J. Tsai and K. D. Jordan, ——ibid. 95, No. 5, 3850–3853 (1991).
P. L. M. Plummer and T. S. Chen, ——ibid. 87, No 5, 4190–4197 (1983).
G. G. Malenkov, M. M. Frank-Kamenetskii, and A. G. Krivtsov,Zh. Strukt. Khim.,28, No. 2, 81–85 (1987).
G. G. Malenkov, A. V. Teplukhin, and V. I. Poltev, ——ibid. 30, No. 4, 89–97 (1989).
V. I. Poltev, A. V. Teplukhin, and G. G. Malenkov,Int. J. Quant. Chem.,42, 1499–1514 (1992).
G. G. Malenkov, inThe Chemical Physics of Solvation, Part A, Elsevier, Amsterdam (1985), pp. 355–389.
G. Brink and L. Glasser,South Afr. J. Chem.,38, No. 1, 31–34 (1985).
E. N. Brodskaya and A. I. Rusanov,Kolloid. Zh.,48, No. 1, 3–11 (1986).
M. V. Kirov,Zh. Strukt. Khim.,35, No. 1, 64–70 (1994).
Additional information
Puebla Autonomous University, Puebla, Mexico, Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences. Institute of Mathematical Problems of Biology, Russian Academy of Sciences. Institute of Physical Chemistry, Russian Academy of Sciences Translated fromZhurnal Strukturnoi Khimii, Vol. 35, No. 6 pp. 113–121, November–December, 1994.
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Gonzalez, E.H., Poltev, V.I., Teplukhin, A.V. et al. Structure and some properties of small water clusters. J Struct Chem 35, 851–858 (1994). https://doi.org/10.1007/BF02578117
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02578117