Abstract
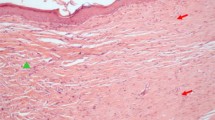
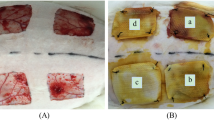
Few details are available on the heterogeneity of glycosaminoglycans (GAGs) in healing fetal wound tissue. We used a sensitive assay for hexosamines to examine changes occurring in the development of normal sheep skin and of wound healing tissue in PVA sponges inserted subcutaneously at different stages of gestation. It was assumed that glucosamine was derived mainly from hyaluronan and galactosamine mainly from dermatan sulphate and chondroitin sulphate. Hexosamine-containing tissue infiltrating the sponges was deposited more repidly in the first week than in the second week. Three days after wounding, approximately 70% of the total GAGs in wound tissue was hyaluronan. The proportion of hyaluronan then fell progressively and by the 14th day contributed 57% to total GAGs. In uninjured skin the contribution of hyaluronan to the total GAGs fell progressively with increasing fetal maturity, the level being 70% at 75 days gestation, but only 35–40% in newborn or adult skin. At no stage of development was there a sudden change in GAG composition suggestive of a transition from regeneration to scar formation. It is concluded that hyaluronan may play an important role in the biochemical sequence leading to collagen fibrillogenesis and mature scar formation.
Similar content being viewed by others
References
Adzick NS, Harrison MR, Glick PL, Beckstead JH, Villa RL, Scheuenstuhl H, Goodson WH III (1985) Comparison of fetal, newborn and adult wound healing by histologic, enzyme histochemical, and hydroxyproline determinations. J Pediatr Surg 20:315–319
Amiel D, Ishizue K, Billings E, Wiig M, Vande Berg J, Akeson WH, Gelberman R (1989) Hyaluronan in flexor tendon repair. J Hand Surg 14A:847–843
Burd DAR, Longaker MT, Adzick NS, Harrison MR, Ehrlich HP (1990) Foetal wound healing in a large animal model: the deposition of collagen is confirmed. Br J Plast Surg 43:571–577
Burrington JD (1971) Wound healing in the foetal lamb. J Pediatr Surg 6:523–528
Chiu ES, Longaker MT, Adzick NS, Stern M, Harrison MR, Stern R (1990) Hyaluronic acid patterns in fetal and adult, wound fluid. Surg Forum 41:636–639
Freund RM, Garg HG, Longaker MT, Siebert JW (1991) Hyaluronan and its associated protein: towards scarless bealing. Surg Forum 42:663–665
Gottschalk A (ed) (1972) Glycoproteins, 2nd edn. Elsevier, Amsterdam
Hallock GG (1985) In utero cleft lip repair in A/J mice. Plast Reconstr Surg 75:785–788
Hellstrom S, Laurent C (1987) Hyaluronan and healing of tympanic membrane perforations. An experimental study. Acta Otolaryngol [Suppl] (Stockh) 442:54–61
Hess A (1954) Reactions of mammalian fetal tissue to injury. II. Skin. Anat Rec 119: 435–448
Horne RSC, Hurley JV, Crowe DM, Ritz M, O'Brien BMcC, Arnold LI (1992) Wound healing in foetal sheep: a histological and electron microscopic study. Br J Plast Surg 45: 333–344
Knight KR, Lepore DA, Horne RSC, Ritz M, Hurley JV, Kumta S, O'Brien BMcC (1993) Collagen content of uninjured skin and scar tissue in foetal and adult sheep. Int J Exp Pathol 74:583–591
Krummel TM, Nelson JM, Diegelmann RF, Lindblad WJ, Salzberg AM, Greenfield LJ, Cohen IK (1987) Fetal response to injury in the rabbit. J Pediatr Surg 22:640–644
Longaker MT, Adzick WS (1991) The biology of fetal wound healing: a review. Plast Reconstr Surg 87:788–798
Longaker MT, Harrison MR, Crombleholme TM, Langer JC, Decker M, Verrier ED, Spendlove R, Stern R (1989) Studies in fetal wound healing. I. A factor in fetal serum that stimulates deposition of hyaluronic acid. J Pediatr Surg 24:789–792
Longaker MT, Adzick NS, Hall JL, Stair SE, Crombleholme TM, Duncan BW, Bradley SM, Harrison MR, Stern R (1990) Studies in fetal wound healing. VII. Fetal wound healing may be modulated by hyaluronic acid stimulating activity in amniotic fluid. J Pediatr Surg 25:430–433
Longaker MT, Bouhana KS, Roberts AB, Harrison MR, Adzick NS, Banda MJ (1991) Regulation of fetal wound healing. Surg Forum 42:654–655
Mast BA, Diegelmann RF, Krummel TM, Cohen IK (1992) Scarless wound healing in the mammalian fetus. Surg Gynecol Obstet 174:441–451
Robinson BW, Goss AN (1981) Intrauterine healing of fetal rat cheek wounds. Cleft Palate J 18:251–255
Roden L (1980) Structure and metabolism of connective tissue proteoglycans. In: Lennarz WJ (ed) The biochemistry of glycoproteins and proteoglycans. Plenum Press, New York, pp 267–380
Rowsell AR (1984) The intrauterine healing of fetal muscle wounds: experimental study in the rat. Br J Plast Surg 37:635–642
Scott JE, Hughes EW (1986) Proteoglycan-collagen relationships in developing chick and bone tendons: influence of the physiological environment. Connect Tissue Res 14: 267–278
Somasundaram K, Prathap K (1970) Intra-uterine healing of skin wounds in rabbit foetuses. J Pathol 100:81–86
Somasundaram K, Prathap K (1972) The effect of exclusion of amniotic fluid on intra-uterine healing of skin wounds in rabbit foetuses. J Pathol 107:127–130
Swann DA, Garg HG, Jung W, Hermann AB (1985) Studies on human scar, tissue proteoglycans. J Invest Dermatol 84:527–531
Swann DA, Garg HG, Hendry CJ, Hermann H, Siebert E, Sotman S, Stafford W (1988) Isolation and partial characterisation, of collagen. Collagen Rel Res 8:295–313
Toole BP, Gross J (1971) The extraacellular matrix of the regenerating newt limb: synthesis and removal of hyaluronate prior to differentiation. Dev Biol 25:57–77
Whitby DJ, Ferguson MWJ (1991) The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development 112:657–668
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Knight, K.R., Horne, R.S.C., Lepore, D.A. et al. Glycosaminoglycan composition of uninjured skin and of scar, tissue in fetal, newborn and adult sheep. Res. Exp. Med. 194, 119–127 (1994). https://doi.org/10.1007/BF02576372
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02576372