Abstract
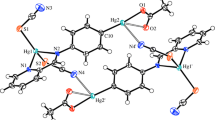
Three new mercury(II) complexes containing tertiary phosphine betaine ligands Ph3P+(CH2)2CO2 − and Ph3P+(CH2)3CO2 − have been synthesized and fully characterized by single-crystal X-ray analysis: [HgCl2{Ph3(CH2)2CO2}],1, space groupP21/n,a=9.819(2),b=14.966(4),c=14.973(5) Å, β=105.67(2)° andZ=4; [HgI2{Ph3(CH2)2CO2}],2,P21/n,a=10.206(2),b=14.807(3),c=15.557(3) Å, β=107.11(2)° andZ=4; [HgCl(μ-Cl){Ph3P(CH2)3CO2}]2,3,\(P\bar 1\),a=10.813(2),b=11.975(3),c=11.180(2) Å, α=87.04(2), β=75.14(1), γ=81.95(1)° andZ=1. The isomorphous complexes1 and2 contain discrete mononuclear molecules in which the mercury(II) atom is unsymmetrically chelated by a Ph3P+(CH2)2CO −2 ligand and coordinated by a pair of terminal halo ligands in a distorted tetrahedral environment, while3 consists of discrete centrosymmetric dinuclear molecules in which the betaine ligand Ph2P+(CH2)3CO −2 acts in the chelate mode and the mercury(II) atoms are unsymmetrically bridged by a pair of chloro ligands.
Similar content being viewed by others
References
Cotton, F.A.; Wilkinson, G.Advanced Inorganic Chemistry (fifth edition); John Wiley and Sons: New York, 1988, pp 620–622.
Wardell J.L. InComprehensive Organometallic Chemistry, Vol. 2, Wilkinson, G.; Gordon F.; Stone, A.; Abel E.W., Eds.; Pergamon Press: Oxford, 1982. Ch. 17, pp 863–978.
Bach, R.D.; Woodard, R.A.; Anderson, T.J.; Glick, M.D.J. Org. Chem. 1982,47, 3707. chandra, G.; Devaprahakara, D.; Muthana, M.S.Curr. Sci. 1971,40, 400.
Grisgin, Yu. K.; Bazhenov, D.V.; Ustynyuk, Yu.A.; Zefirrov, N.S.; Kartashov, V.R.; Sokolova, T.N.; Skorobogatova, E.V.; Chernov, A.N.Tetrahedron Lett. 1988,29, 4631. (b) Larock, R.C.; Oertle, K.; Beatty, K.M.J. Am. Chem. Soc. 1980,102, 1966.
Kamenar, B.; Penavic, M.Inorg. Chim. Acta 1972,6, 191.
Grdenić, D.; Kamenar, B.; Korpar-Colig, B.; Sikirica, M.; Jovanovski, G.J. Chem. Soc. Chem. Commun. 1974, 646.
Grdenić, D.; Sikirica, M.Z. Crystallography 1979,150, 107.
Allman, R.; Flatan, K.; Musso., H,Chem. Ber. 1973,137, 366.
Robert, P.J.; Ferguson, G.; Goel, R.G.; Ogini, O.W.; Restivo, R.J.J. Chem. Soc. Dalton Trans. 1978, 253.
Canty, A.J.; Raston, C.L.; White, A.H.Aust. J. Chem. 1979,32, 311.
Lau, W.; Huffman, J.C.; Kochi, J.K.,J. Am. Chem. Soc. 1982,104, 5515.
Chen, X.-M.; Mak, T.C.W.J. Chem. Soc. Dalton Trans. 1992, 1585.
Bradley, D.C.; Mehrotra, R.C.; Gaur, D.P.Metal Alkoxides; Academic Press: New York, 1978.
Chow, M.-X.; Chen, X.-M.; Mak, T.C.W.J. Chem. Soc. Dalton Trans. 1993, 3413.
Chen, X.-M.; Mak, T.C.W.Inorg. Chim. Acta. 1991,182, 139.
Chen, X.-M.; Mak, T.C.W.Polyhedron 1991,10, 273.
Chen, X.-M.; Mak, T.C.W.J. Cryst. Spectrosc. Res. 1991,21, 21.
Chen, X.-M.; Mak, T.C.W.Aust. J. Chem. 1991,44, 1783.
Chen, X.-M.; Mak, T.C.W.J. Chem. Soc. Dalton Trans. 1991, 1219.
Chen, X.-M.; Mak, T.C.W.Polyhedron 1991,10, 1723.
Li, S.-L. Mak, T.C.W.J. Chem. Soc. Dalton Trans. 1995, 1519.
Denney, D.B., Smith, L.C.J. Org. Chem. 1962,27, 3404.
Sparks, R.A. InCrystallographic Computing Techniques; Ahmed, R.F.; Ed.; Munksgaard: Copenhagen, 1971, p 452
Sheldrick, G.M. InComputational Crystallography; Sayre, D., Ed.; Oxford University Press: New York, 1982, pp 506–514.
International Tables for X-Ray Crystallography Kynoch Press: Birmingham (now distributed by Kluwer Academic Press, Dordrecht), 1974, Vol. 4, pp 55, 99, 149.
Grdenić, D.Quart. Rev. Chem. Soc. London 1969,19, 303.
Dean, P.A.W.Prog. Inorg. Chem. 1978,24, 109.
Bell, N.A.; Goldsstein, M.; Jones, T.; Nowell, I.W.Inorg. Chim. Acta 1981,48, 185. (b) Bell, N.A.; Dee, T.D.; Goldstein, M.; Jones, T.; March, L.A.; Nowell, I.W.ibid. Inorg. Chim. Acta 1982,61, 83. (c) Bell, N.A.; Dee, T.D.; Goldstein, M.; Nowell, I.W.ibid. Inorg. Chim. Acta 1983,70, 215. (d) Bell, N.A.; March, L.A.; Nowell, I.W.ibid. Inorg. Chim. Acta 1989,156, 201. (e) Bell, N.A.; March, L.A.; Nowell, I.W.ibid. Inorg. Chim. Acta 1989,162, 57.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, SL., Mak, T.C.W. Interaction of mercury(II) halides with tertiary phosphine betaines: synthesis and structural characterization of [HgX2{Ph3P(CH2)2CO2}] (X=Cl, I) and [HgCl(μ-Cl)-{Ph3P(CH2)3CO2}]2 . J Chem Crystallogr 27, 91–97 (1997). https://doi.org/10.1007/BF02575901
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02575901