Abstract
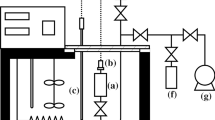
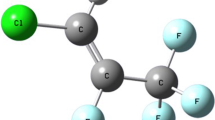
The effect of pressure on the volume of R141, R131, and R132b is reported as volume ratios (the volume under pressure relative to its value at atmospheric pressure) at six temperatures covering the range 278.15 to 338.13 K and pressures up to 380 MPa for R141 and R131a. For R132b the same temperature range has been used, but above its normal boiling point experimental arrangements have limited maximum pressures to below 300 MPa, with some loss of accuracy. Densities have been measured at atmospheric pressure for each liquid. The experimental data have been used to calculate isothermal compressibilities, thermal expansivities, and internal pressures: the change in isobaric heat capacity from its value at atmospheric pressure has also been estimated. The volume ratios for all three compounds can be represented by a version of the Tait equation based on previously reported data for 1,2-dicloroethane and 1,1,2-trichloroethane with the inclusion of allowances for the substitution in the former of chlorine or fluorine for the hydrogens on one of the carbons.
Similar content being viewed by others
References
See e.g., R. Malhotra and L. A. Woolf,High Temp. High Press. 25:179 (1993).
R. Malhotra and L. A. Woolf,Fluid Phase Equil. 94:227 (1994).
S. J. Ashcroft, D. R. Booker, and J. C. R. Turner,J. Chem. Soc. Faraday Trans. 86:145 (1990).
R. Malhotra and L. A. Woolf,Int. J. Thermophys. 14:1021 (1993).
R. Malhotra, W. E. Price, L. A. Woolf, and A. J. Easteal,Int. J. Thermophys. 11: 835 (1990). [TheB’s forC=0.21 are 127.80 MPa at 278.15 K, 118.68 (288.15), 110.24 (298.14), 98.23 (313.14), 91.08 (323.14), and 80.57 (338.13).]
J. A. Riddick W. B. Bunger, and T. K. Sakano,Organic Solvents, Physical Properties and Methods for purification, 4th ed. (Wiley-Interscience, New York, 1986).
R. C. Reid, J. M. Prausnitz, and B. E. Poling,The Properties of Gases and liquids, 4th ed. (McGraw-Hill, New York, 1987).
A. Kumagai,Int. J. Thermophys. 10:1229 (1989). (These data are represented for aC of 0.21, usingT c =602 K, byB=−133.404+130.187/T r, with a rmsd of 0.46.)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Malhotra, R., Woolf, L.A. Volumetric properties under pressure for 1,2-dichlorofluoroethane (R141), 1-fluoro-1,1,2-trichloroethane (R131a), and 1,2-dichloro-1,1-difluoroethane (R132b). Int J Thermophys 18, 49–63 (1997). https://doi.org/10.1007/BF02575201
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02575201