Summary
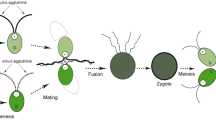
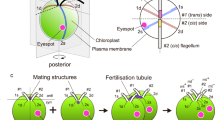
Mutant strains of the unicellular green algaChlamydomonas eugametos are described which are defective in sexual fusion. All mutants are mating type plus (mt+). They are unable to fuse because none of them is capable of protruding a mating structure through the cell wall, neither during sexual agglutination nor after adding dibutyryl-cAMP or compounds that raise the intracellular calcium level, treatments that are effective in wild type cells. Evidence is presented that these mutants lack the lytic enzyme activity which is normally involved in the local hydrolysis of the cell wall to allow the protrusion of the mating structure. Furthermore, a simple light microscopic method is presented to determine the presence of activated mating structures.
Similar content being viewed by others
Abbreviations
- db-cAMP:
-
dibutyryl cyclic AMP
- Mab:
-
monoclonal antibody
- mt+ :
-
mating type plus
- mt− :
-
mating type minus
- WGA:
-
wheat-germ agglutinin
References
Bloodgood RA, Levin EN (1983) Transient increase in calcium efflux accompanies fertilization inChlamydomonas. J Cell Biol 97: 397–404
—, Salomonsky NL (1990) Calcium influx regulates antibody induced gylcoprotein movements within theChlamydomonas flagellar membrane. J Cell Sci 96: 27–33
Brown Jr RM, Johnson C, Bold HC (1986) Electron and phasecontrast microscopy of sexual reproduction inChlamydomonas moewusii. J Physcol 4: 100–120
Buchanan MJ, Snell WJ (1988) Biochemical studies on lysin, a cell wall degrading enzyme released during fertilization inChlamydomonas Exp Cell Res 179: 181–193
—, Imam SH, Eskue WA, Snell WJ (1989) Lysin, during sexual signalling inChlamydomonas: the enzyme is stored as an inactive, higher relative molecular mass precursor in the periplasm. J Cell Biol 108: 199–207
Claes H (1980) Calcium ionophore-induced stimulation of secretory activity inChlamydomonas reinhardtii. Arch Microbiol 124: 81–86
Crabbendam KJ, Nanninga N, Musgrave A van den Ende H (1984) Flagellar tip activation in vis-à-vis parirs ofChlamydomonas eugametos. Arch Microbiol 138: 220–223
Demets R, Tomson AM, Homan WL, Stegwee D, van den Ende H (1988) Cell-cell adhesion in conjugatingChlamydomonas gametes: a self-enhancing process. Protoplasma 145: 27–36
Detmers PA, Goodenough UW, Condeelis J (1983) Elongation of the fertilization tubule inChlamydomonas: new observations on the core microfilaments and the effect of transient intracellular signals on their structure integrity. J Cell Biol 97: 522–532
Elzenga JTM, Nanninga N, Lens P, van den Ende H (1982) Flagellar tip activation inChlamydomonas eugameto. FEMS Microbiol Lett 15: 279–283
Forest CL (1987) Genetic control of plasma membrane adhesion and fusion inChlamydomonas gametes. J Cell Sci 88: 613–621
Goodenough UW (1980) Ionophore simulation of mating signals inChlamydomonas. J Cell Biol 87: 37a
— Hwang C, Martin H (1976) Isolation and genetic analysis of mutant strains ofChlamydomonas reinhardi defective in gametic differentiation Genetics 82: 169–186
— Detmers PA, Hwang C (1982) Activation for cell fusion inChlamydomonas: analysis of wild-type gametes and nonfusion mutants. J Cell Biol 92: 378–386
Homan WL, Musgrave A, Molenaar EM, van den Ende H (1980) Isolation of monovalent sexual binding components fromChlamydomonas eugametos flagellar membranes, Arch Microbiol 128: 120–125
—, Signon C, van den Briel W, Wagter R, de Nobel H, Mesland D, Musgrave A, van den Ende H (1987a) Transport of membrane receptors and the mechanics of sexual cell fusion inChlamydomonas eugametos. FEBS Lett 215: 323–326
—, Kalshoven H, Kolk AHJ, Musgrave A, Schuring F, van den Ende H (1987b) Monoclonal antiboddies to surface glycoconjugates inChlamydomonas eugametos recognize strain-specificO-methyl sugars. Planta 170: 328–335
—, Musgrave A, de Nobel H, Wagter R, de Wit D, Kolk A, van den Ende H (1988) Monoclonal antibodies directed against the sexual binding site ofChlamydomonas eugametos gametes. J Cell Biol 107: 177–191
Kalshoven HW, Musgrave A, van den Ende H (1990) Mating receptor complex in the flagellar membrane ofChlamydomonas eugametos gametes. Sex Plant Reprod 3: 77–87
Kaska DD, Piscopo IC, Gibor A (1985) Intracellular calcium redistribution during mating inChlamydomonas reinhardtii. Exp Cell Res 160: 371–379
Klis FM, Samson MR, Touw E, Musgrave A van den Ende H (1985) Sexual agglutination in the unicellular green algaChlamydomonas eugametos. Identification and properties of the mating type plus agglutination factor. Plant Physiol 79: 740–745
Kolk AHJ, Ho ML, Klatser PR, Eggelte TA, Kuyper S, de Jonge S, van Leeuwen J (1984) Production and characterization of monoclonal antibodies toMycobacterium tuberculosis, M. bovis (BCG) andM. leprae. Clin Exp Immunol 58: 511–521
Kooijman R, Elzenga TJM, de Wildt P, Musgrave A, Schuring F, van den Ende H (1986) Light dependence of sexual agglutinability inChlamydomonas eugametos. Planta 169: 370–378
—, de Wildt P, Beumer S, van Vliet G, Homan W, Kalshoven H, Musgrave A, van den Ende H (1989) Wheat germ agglutinin induces mating reactions inChlamydomonas eugametos by cross linking agglutinin-associated glycoproteins in the flagellar membrane. J Cell Biol 109: 1677–1687
——, van den Briel W, Tan S, Musgrave A, van den Ende H (1990) Cyclic AMP is one of the intracellular signals during the mating ofChlamydomonas eugametos. Planta 181: 529–537
Martin NC, Goodenough UW (1975) Gametic differentiation inChlamydomonas reinhardtii. I. Production of gametes and their fine structure. J Cell Biol 67: 587–605
Matsuda Y, Saito T, Yamaguchi T, Kawasa H (1985) Cell wall lytic enzyme relased by mating gametes ofChlamydomonas reinhardtii is a metalloprotease and digests the sodium perchlorate-insoluble component of cell wall. J Biol Chem 260: 6373–6377
———, Koseki M, Hayashi K (1987) Topography of cell wall lytic enzyme inChlamydomonas reinhardtii: form and location of the stored enzyme in vegetative cell and gamete. J Cell Biol 104: 321–329
Mesland DAM (1976) Mating inChlamydomonas eugametos: A scanning electron microscopical study. Arch Microbiol 109: 31–35
—, Hoffman JL, Caligor E, Goodenough UW (1980) Flagellar tip activation stimulated by membrane adhesions inChlamydomonas eugametos. J Cell Biol 84: 599–617
Musgrave A, Homan W, van den Briel W, Lelie N, Schol D, Ero L, van den Ende H (1979) Membrane glycoproteins ofChlamydomonas eugametos flagella. Planta 145: 417–425
—, van Eijk E, te Welscher R, Broekman R, Lens P, Homan W, van den Ende H (1981) Sexual agglutination factor fromChlamydomonas eugametos. Planta 153: 362–369
—, de Wildt P, Broekman R, van den Ende H (1983) The cell wall ofChlamydomonas eugametos immunological aspects. Planta 158: 82–89
——, Schuring F, Crabbendam K, van den Ende H (1985) Sexual agglutination inChlamydomonas eugametos before and after cell fusion. Planta 166: 234–243
Pasquale SM, Goodenough UW (1987) Cyclic AMP functions as a primary sexual signal in gametes ofChlamydomonas reinhardtii. J Cell Biol 105: 2279–2292
Pijst HLA, van Driel R, Janssens PMW, Musgrave A, van den Ende H (1984) Cyclic AMP is involved in sexual reproduction ofChlamydomonas eugametos. FEBS Lett 174: 132–136
Samson MR, Klis FM, van den Ende H (1988) Cysteine as a fusion inhibitor in sexual mating reaction of the green algaChlamydomonas eugametos. Acta Bot Neerl 37: 351–361
Schuring F, Smeenk JW, Homan WL, Musgrave A, van den Ende H (1987) Occurrence ofO-methylated sugars in surface glycoconjugates inChlamydomonas eugametos. Planta 170: 322–327
—, Brederoo J, Musgrave A, van den Ende H, (1990) Increase in calcium triggers mating structure activation inChlamydomonas eugametos. FEMS Microbiol Lett 71: 237–240
Snell WJ, Buchanan M, Clausell A (1982) Lidocaine reversibly inhibits fertilization inChlamydomonas: a possible role in sexual signaling. J Cell Biol 94: 607–612
—, Eskue W, Buchanan MJ (1989) Regulated secretion of a serine protease that activates an extracellular matrix-degrading metalloprotease during fertilization inChlamydomonas. J Cell Biol 109: 1689–1694
Tilney LG, Inoué S (1985) Acrosomal reaction of the Thyone sperm. III. The relationship between actin assembly and water influx during the extension of the acrosomal process. J Cell Biol 100: 1273–1283
Tomson AM, Demets R, Signon CAM, Stegwee D, van den Ende H (1986) Cellular interactions during the mating process inChlamydomonas eugametos. Plant Physiol 81: 522–526
Triemer RE, Brown Jr RM (1975a) The ultrastructure of fertilization inChlamydomonas moewusii. Protoplasma 84: 315–325
—— (1975a) Fertilization inChlamydomonas reinhardi, with special reference to the structure, development, and fate of the choanoid body. Protoplasma 85: 99–107
Wiese L (1965) On sexual agglutination and mating-type substances (gamones) in isogamous heterothallicChlamydomonads. I. Evidence of the identity of the gamones with the surface components responsible for sexual flagellar contact. J Phycol 1: 46–54
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schuring, F., Musgrave, A., Elders, M.C.C. et al. Fusion-defective mutants ofChlamydomonas eugametos . Protoplasma 162, 108–119 (1991). https://doi.org/10.1007/BF02562554
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02562554