Summary
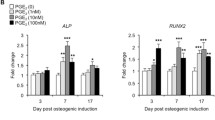
The effects of prostaglandin E2(PGE2) on DNA and collagen synthesis in two separate cell populations were investigated. In view of their morphology, ALPase activity, DNA and collagen synthesis, and response to PGE2, one population was in an undifferentiated state consisting of preosteoblast-like (PL) cells and the other was in a differentiated state consisting predominantly of osteoblastlike (OB) cells. As parameters of bone-forming activity, the incorporation of3H-thymidine into DNA and the incorporation of3H-proline into collagenase digestible protein were measured to assess DNA and collagen synthesis. The cells were treated with PGE2 in the presence of indomethacin (IM) to avoid the influence of endogenous prostaglandins. At 24 hours, IM stimulated the DNA synthesis in both cell populations. Furthermore, a greater stimulation was found in the PL cells than in the OB cells. On the other hand, exogenously supplemented PGE2 reversed the IM-induced stimulation of DNA synthesis. In contrast, high concentrations of PGE2 alone increased the DNA synthesis. With respect to collagen synthesis, IM showed an inhibitory effect, especially in the PL cells. This inhibitory effect was also reversed by the addition of PGE2. In addition to the stimulation of collagen synthesis, PGE2 enhanced the proportion of protein synthesized as collagen. In the PL cells, the percentage of collagen synthesis was markedly decreased when cultured with IM for 48 hours. These results suggested that the effects of IM were mediated in part via its ability to reduce biosynthesis of prostaglandins, and that PGE2 is a multifunctional autocrine regulator of bone formation.
Similar content being viewed by others
References
Ueda K, Saito A, Nakano H, Aoshima M, Yokoto M, Muraoka R, Iwaya T (1980) Cortical hyperostosis following long-term administration of prostaglandin, E1 in infants with cyanotic congenital heart disease. J Pediatr 97:834–836
Ringel RE, Brenner JI, Haney PJ, Burns JE, Molton AL, Berman MA (1982) Prostaglandin-induced periostitis: a complication of long-term PG E1 infusion in an infant with congenital heart disease. Radiology 142:657–658
Sone K, Tashiro M, Fujinaga T, Tomomasa T, Tokuyama K, Kuroume T (1982) Long-term low-dose prostaglandin, E1 administration (Letter) J Pediatr 97:866–867
Jee WSS, Ueno K, Deng YP, Woodbury DM (1985) The effects of prostaglandin E2 in growing rats: increased metaphyseal hard tissue and cortico-endosteal bone formation. Calcif Tissue Int 37:148–157
Jee WSS, Ueno K, Kimmel DB, Woodbury DM, Price P, Woodbury LA (1987) The role of bone cells in increasing metaphyseal hard tissue in rapidly growing rats treated with prostaglandin E2 Bone 8:171–178
Ueno K, Kimmel DB, Haba T, Jee WSS (1984) Increased metaphyseal hard tissue mass in growing long bone following prostaglandin E2 administration. In: Cohn DV, Fujita T, Poots JT, Talmage RV (eds) Endocrine control of bone and calcium metabolism. Elsevier Science Publishers BV, Amsterdam, pp 151–154
Ueno K, Haba T, Woodbury DM, Price P, Anderson R, Jee WSS (1985) The effect of prostaglandin E2 in rapidly growing rats: depressed longitudinal and radial growth and increased metaphyseal hard tissue mass. Bone 6:79–86
Furuta Y, Jee WSS (1986) Effect of 16,16-dimethyl prostaglandin E2 methyl ester on weanling rat skeleton: daily and systemic administration. Anat Rec 215:305–316
Blumenkrantz N, Søndergaard J (1971) Effect of prostaglandin E1 and F1α on biosynthesis of collagen. Nature New Biol 239:246
Chyun YS, Raisz LG (1984) Stimulation of bone formation by prostaglandin E2 Prostaglandins 27:96–103
Nefussi JR, Baron R (1985) PG E2 stimulates both resorption and formation of bone in vitro: differential responses of the periosteum and the endosteum in fetal rat long bone cultures. Anat Rec 211:9–16
Martin TJ, Livesey SA, Partridge NC, Zajac JD, Ng KW (1984) Effects of PTH and PG E2 upon cAMP-dependent protein kinase isoenzymes and cell proliferation in osteoblasts. In: Cohn DV, Fujita T, Potts JT, Talmage RV (eds) Endocrine control of bone and calcium metabolism. Elsevier Science Publishers BV, Amsterdam, pp 159–162
Feyen JHM, Bon AD, Plas A, Lowik CWGM, Nijiweide PJ (1981) Effects of exogenous prostanoids on the proliferation of osteoblast-like cells in vitro. Prostaglandins 30:827–840
Plas A, Feyen JHM, Nijiweide PJ (1985) Direct effect of parathyroid hormone on the proliferation of osteoblast-like cells: a possible involvement of cyclic AMP. Biochem Biophys Res Commun 129:918–925
Hakeda Y, Yoshino T, Nakatani Y, Kurihara N, Maeda N, Kumegawa M (1986) Prostaglandin E2 stimulates DNA synthesis by a cyclic AMP-independent pathway in osteoblastic clone MC3T3-E1 cells. J Cell Physiol 28:155–161
Livesey SA, Kemp BE, Re CA, Partridge NC, Martin TJ (1982) Selective hormonal activation of cyclic AMP-dependent protein kinase isoenzymes in normal and malignant osteoblasts. J Biol Chem 257:14983–14987
Johnson LC (1964) Morphologic analysis in pathology: the kinetics of disease and general biology of bone. In: Frost HM (ed) Bone biodynamics. Little, Brown and Co., Boston, pp. 543–654
Nolan RD, Partridge NC, Godfrey HM, Martin TJ (1983) Cyclo-oxygenase products of arachidonic acid metabolism in rat osteoblasts in culture. Calcif Tissue Int 35:294–297
Partridge NC, Hillyard CJ, Nolan RD, Martin TJ (1985) Regulation of prostaglandin production by osteoblast-rich calvarial cells. Prostaglandins 30:527–539
Yokota K, Kusaka M, Ohshima T, Yamamoto S, Kurihara N, Yoshino T, Kumegawa M (1986) Stimulation of prostaglandin E2 synthesis in cloned osteoblastic cells of mouse (MC3T3-E1) by epidermal growth factor. J Biol Chem 261:15410–15415
Rodan SB, Rodan GA, Simmons HA, Walenga RW, Feinstein MB, Raisz LG (1981) Bone resorptive factor produced by osteosarcoma cells with osteoblastic features is PG E2. Biochem Biophys Res Commun 102:1358–1365
Boonekamp PM, Hekkelman JW, Hamilton JW, Cohn DV, Jilka RL (1984) Effect of culture on the hormone responsive-ness of bone cells isolated by an improved sequential digestion procedure. Biochemistry, Proceedings B 87:371–380
Lowry OH, Roberts NR, Wu ML, Hixon WS, Crawford EJ (1954) The quantitative histochemistry of brain II. Enzyme measurements. J Biochem 207:19–37
Hausamen TU, Helger R, Rick W, Gross W (1967) Optimal conditions for the determination of serum alkaline phosphatase by a new kinetic method. Clin Chim Acta:214–245
Burton K (1956) A study of the conditions and mechanism of the diphenylamine reaction for the colormetric estimation of deoxyribonucleic acid. Biochem J 62:315–323
Peterkofsky B, Diegelman R (1971) Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry 10:988–994
Peterkofsky B (1972) The effectt of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys 152:318–328
Gray JC, Elves MW (1982) Donor cell's contribution to osteogenesis in experimental cancellous bone grafts. Clin Orthop Rel Res 163:261–271
Volpi G, Palazzini S, Cané V, Remaggi F, Muglia MA (1981) Morphometric analysis of osteoblast dynamics in the chick embryo tibia. Anat Embryol 162:393–401
Raisz LG, Koolemans-Beynen AR (1974) Inhibition of bone collagen synthesis by prostaglandin E2 in organ culture. Prostaglandins 8:377–385
Larjava H, Multanen VM, Paunio K (1985) Effects of prostaglandin E2 and dental plaque on bone collagen and hyaluronic acid synthesis. Proc Finn Dent Soc 81:163–170
Hakeda Y, Nakatani Y, Kurihara N, Ikeda E, Maeda N, Kumegawa M (1985) Prostaglandin E2 stimulates collagen and non-collagen protein synthesis and prolyl hydroxylase activity in osteoblastic clone MC3T3-E1 cells. Biochem Biophys Res Commun 126:340–345
Raisz LG, Martin TJ (1983) Prostaglandins in bone and mineral metabolism. In: Peck WA (ed) Bone and mineral research, Annual 2. Elsevier Science Publishers B.V., Amsterdam, pp 286–310
Lee PC, Radloff D, Schweppe JS, Jungmann RA (1976) Testicular protein kinases: characterization of multiple forms and ontogeny. J Biol Chem 251:914–921
Byus CV, Chubb JM, Huxtable RJ, Russel DH (1976) Increase in type I adenosine 3′, 5′-monophosphate-dependent protein kinase during isoproterenol-induced cardiac hypertrophy. Biochem Biophys Res Commn 73:694–702
Costa M, Gerner EW, Russell DH (1978) Cyclic AMP levels and types I and II cyclic AMP-dependent protein kinase activity in synchronized cells and in quiescent cultures stimulated to proliferate. Biochem Biophys Acta 538:1–10
Haddox MK, Magun BE, Russell DH (1980) Differential expression of type I and type II cyclic AMP-dependent protein kinases during cell cycle and cyclid AMP-induced growth arrest. Proc Natl Acad Sci USA 77:3445–3449
Cho-Chung YS (1980) Cyclic AMP and its receptor protein in tumor growth regulation in vivo. J Cyclic Nucleotide Res 6:163–177
Hakeda Y, Nakatani Y, Hiramatu M, Kurihara N, Tsunoi M, Ikeda E, Kumegawa M (1985) Inductive effects of prostaglandins on alkaline phosphatase in osteoblastic cells, clone MC3T3-E1. J Biochem 97:97–104
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nagai, M. The effects of prostaglandin E2 on DNA and collagen synthesis in osteoblastsIn Vitro . Calcif Tissue Int 44, 411–420 (1989). https://doi.org/10.1007/BF02555970
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02555970