Abstract
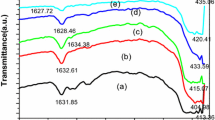
TG-DTA-EGA studies have shown that anhydrous uranyl nitrate cannot be obtained by thermal decomposition of uranyl nitrate hexahydrate. Hydrolysis and polymerization of the salt during dehydration resulted in hydroxynitrates which decomposed in multiple steps with the evolution of oxides of nitrogen and water. The extent of hydrolysis dependend on the sample size, heating rate and nature of sample containment. Large samples on decomposition at relatively high heating rates showed evolution of nitric oxide even above 500°C. Infrared studies on the residues prepared at various temperatures supported the conclusions.
Zusammenfassung
TG-DTA-EGA-Untersuchungen erwiesen, daß wasserfreies Uranylnitrat nicht durch die thermische Zersetzung von Uranylnitrathexahydrat erhalten werden kann. Während der Dehydratation stattfindende Hydrolyse und Polymerisation des Salzes führen zu Hydroxynitraten, die sich in mehreren Schritten unter Freisetzung von Stickoxiden und Wasser zersetzen. Das Ausmaß der Hydrolyse hing von Probengröße, Aufheizgeschwindigkeit und Art der Probenkontrolle ab. Große Proben zeigten bei der Zersetzung bei relativ, hohen Aufheizgeschwindigkeiten die Freisetzung von Stickstoffmonoxid sogar oberhalb 500°C. Die Schlußfolgerungen wurden durch IR-Untersuchungen der bei verschiedenen Temperaturen erhaltenen Rückstände bestätigt.
Similar content being viewed by others
References
R. S. Ondrejcin and T. P. Garrett, Jr., J. Phys. Chem., 65 (1961) 470.
S. Hartland and R. J. Nesbitt, J. Appl. Chem., 14 (1964) 406.
W. Lodding and L. Ojamaa, J. Inorg. Nucl. Chem., 27 (1965) 1261.
W. H. Smith, J. Inorg. Nucl. Chem., 30 (1968) 1761.
F. Weigel, ‘The Chemistry of the Actinide Elements’, 2nd Ed., Ed. J. J. Katz, G. T. Seaborg, and L. R. Morss, Chapmann and Hall, London 1986, Vol. 1, p. 361.
J. L. Woodhead, A. M. Deane, A. C. Fox and J. M. Fletcher, J. Inorg. Nucl. Chem., 28 (1966) 2175.
M. Åberg, Acta Chem. Scand., Ser. A, 32 (1978) 101.
C. C. Addison, H. A. J. Champ, N. Hodge and A. H. Norbury, J. Chem. Soc., (1964) 2354.
Chemical Abst., Vol. 108, 1988, Abst. No. 115138s.
P. V. Ravindran, P. S. Dhami, K. V. Rajagopalan and M. Sundaresan, Thermochim. Acta, 197 (1992) 91.
P. V. Ravindran, J. Rangarajan and A. K. Sundaram, Thermochim. Acta, 147 (1989) 331.
J. C. Taylor and M. H. Mueller, Acta Cryst., 19 (1965) 536.
B. M. Gatehouse and A. E. Comyns, J. Chem. Soc., (1958) 3965.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rajagopalan, K.V., Ravindran, P.V. & Radhakrishnan, T.P. Thermal decomposition of uranyl nitrate hexahydrate. Journal of Thermal Analysis 44, 89–96 (1995). https://doi.org/10.1007/BF02547137
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02547137